1-nitroso-2-naphthol
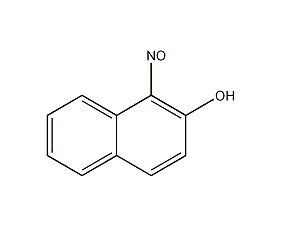
Structural formula
| Business number | 03NA |
|---|---|
| Molecular formula | C10H7NO2 |
| Molecular weight | 173.17 |
| label |
1-Nitroso-2-naphthol, α-nitroso-β-naphthol, cobalt reagent, 1-nitrosonaphthol, Nitroso-beta-naphthol, 1,2-Naphthoquinone-1-oxime, 1-nitros-2-naphthol, a-Nitroso-β-naphthol, aromatic compounds |
Numbering system
CAS number:131-91-9
MDL number:MFCD00003884
EINECS number:205-043-0
RTECS number:QL4725000
BRN number:776947
PubChem number:24847189
Physical property data
1. Properties: Orange-yellow flake or prismatic crystals.
2. Melting point (℃): 109.5
3. Solubility: soluble in alcohol, ether, benzene, carbon tetrachloride, alkali and acetic acid, slightly soluble in cold petroleum Ether, insoluble in water.
4. Stability: flammable, explodes when spontaneously ignited, and will spontaneously ignite quickly when exposed to acid or alkali.
Toxicological data
1. Acute toxicity: Rat oral LD: 500mg/kg
Mouse is the reported exposure route LC5O: 400mg/kg
2. Mutagenicity: Salmonella gene mutation :10ug/plate
Bacillus subtilis DNA repair: 10mmol/L
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 48.28
2. Molar volume (cm3/mol): 135.7
3. Isotonic specific volume (90.2K): 361.9
4. Surface tension (dyne/cm): 50.5
5. Polarizability (10-24cm3): 19.14
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 4
6. Topological molecule polar surface area 49.7
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 195
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The solution is yellow or brown-orange.��With Co3+ , ZrO2+, Fe2+ etc. to generate red or blue microorganisms Complexes soluble in water can be extracted with chloroform or carbon tetrachloride.
Storage method
Stored in a cool, dry place and sealed.
Synthesis method
Introduction to production method
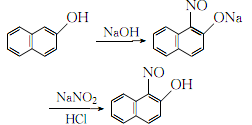
1. Obtained from nitrosation of β-naphthol. Stir β-naphthol and aqueous sodium hydroxide to dissolve. After cooling, add sodium nitrite and filter. The filtrate is cooled to below 0°C. While stirring, the temperature does not exceed 0°C. Continue to add 10% hydrochloric acid dropwise until the Congo red test paper turns blue. After stirring for 0.5 h, filter, wash with water until the chloride ions are no larger than those in tap water, wash once with distilled water and ethanol, filter out the crystals, and then refine to obtain the finished product.
2.Dissolve industrial β-naphthol and sodium hydroxide in a 1:1 (mol) ratio in 50°C water, and cool After reaching 0°C, add 2.5% sodium nitrite solution of the same amount under rapid stirring, keep the temperature at 0°C, continuously add 10% hydrochloric acid dropwise until the Congo red test paper turns blue, stir for another half an hour, let it stand, and filter , the crystals are first washed with water until the C-ion content is no greater than the C-ion content in tap water, and then washed once with distilled water and ethanol respectively. The resulting crystals are added to boiling glacial acetic acid, stirred to dissolve, filtered, and filtered. span style=”font-family:Arial;”>Add ferrous sulfate solution into the solution until the precipitate is no longer formed. After filtering, wash it thoroughly with distilled water until it is qualified. After drying, pure 1-nitroso-2-naphthalene is obtained. phenol.
The process reaction formula is:

Purpose
1. This product can be obtained through sulfonation, diazotization and nitration to obtain 6-nitro-2,1-hydroxynaphthalene diazonium-4-sulfonic acid. Also known as 6-nitro-1,2-diazooxynaphthalene-4-sulfonic acid, it is an intermediate of acid mordant black dye. This product is a reagent for the determination of cobalt, palladium, copper and iron.
2.Used as an intermediate in organic synthesis.
