1,1-Dimethyl-2-phenylethyl acetate
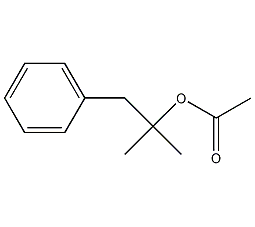

Structural formula
| Business number | 03YL |
|---|---|
| Molecular formula | C12H16O2 |
| Molecular weight | 192.25 |
| label |
Dimethylbenzyl alcohol ethyl ester, Dimethylbenzyl acetate, α,α-dimethylphenylethyl acetate, Benzyldimethylcarbinol acetate, Dimethylbenzylcarbinyl acetate |
Numbering system
CAS number:151-05-3
MDL number:MFCD00026196
EINECS number:205-781-3
RTECS number:SG8100000
BRN number:None
PubChem number:24901042
Physical property data
1. Physical property data:
1.Characteristics: Colorless and transparent liquid with floral and fruity aroma.
2.Density (g/mL, 25/4℃):0.998
3.Refractive index (nD20): 1.4925
4.Flash Point (°F): 206
5.Boiling point (ºC):250
6 .Solubility:Soluble in ethanol, most non-volatile oils and mineral oil, slightly soluble in propylene glycol, insoluble in glycerin and water.
Toxicological data
2. Toxicological data:
1, acute toxicity: rat oral LD50:3300 mg/kgEcological data
3. Ecological data:
Other harmful Effect: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data: 1. Molar refractive index:56.07 2. Molar volume (m3/ mol):191.8 3. Isotonic specific volume (90.2K): 462.8 4. Surface tension (dyne/cm): 33.9 5. Polarizability(10-24cm3):22.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
N>
1. Molar refractive index:56.07
2. Molar volume (m3/ mol):191.8
3. Isotonic specific volume (90.2K): 462.8
4. Surface tension (dyne/cm): 33.9
5. Polarizability(10-24cm3):22.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 2.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 191
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials:Strong oxidizing agentStorage method
Seal and store in a dry and cool place.
Synthesis method
It is produced by direct esterification of dimethylbenzyl alcohol and acetic anhydride.
Purpose
1. GB 2760—96 is specified as Permitted Flavors. It is mainly used to prepare fruit flavors such as pears, cherries, and strawberries.
2. Use Used to prepare a variety of cosmetics, soaps and food flavors.
3. Available To enhance head and body fragrance, it is often used in fragrance formulas of hyacinth, magnolia, lilac, and lily of the valley. In addition, it can also be used in fragrance formulas such as rose, geranium, jasmine, and orchids to enhance the aroma and make it fresh and elegant. Adding fragrance to perfume can give it a floral scent. It can also be used in soap flavors and food flavors.
5. Number of tautomers: None
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 14
8. Surface charge :0
9. Complexity: 191
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials:Strong oxidizing agentStorage method
Seal and store in a dry and cool place.
Synthesis method
It is produced by direct esterification of dimethylbenzyl alcohol and acetic anhydride.
Purpose
1. GB 2760—96 is specified as Permitted Flavors. It is mainly used to prepare fruit flavors such as pears, cherries, and strawberries.
2. Use Used to prepare a variety of cosmetics, soaps and food flavors.
3. Available To enhance head and body fragrance, it is often used in fragrance formulas of hyacinth, magnolia, lilac, and lily of the valley. In addition, it can also be used in fragrance formulas such as rose, geranium, jasmine, and orchids to enhance the aroma and make it fresh and elegant. Adding fragrance to perfume can give it a floral scent. It can also be used in soap flavors and food flavors.
