1,1,1-trifluoro-2,4-pentanedione
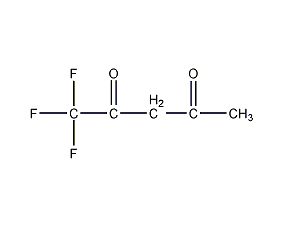

Structural formula
| Business number | 04GT |
|---|---|
| Molecular formula | C5H5F3O2 |
| Molecular weight | 154.09 |
| label |
α,α,α-trifluoroacetylacetone, 1,1,1-trifluoroacetylacetone, α,α,α-Trifluoroacetylacetone, 1,1,1-Trifluoroacetylacetone, Extracting agent, chelating agent |
Numbering system
CAS number:367-57-7
MDL number:MFCD00000427
EINECS number:206-698-5
RTECS number:None
BRN number:1705177
PubChem number:24854129
Physical property data
一 , physical property data
Traits :Colorless or light yellow transparent liquid.
Density (g/mL,25/4℃): 1.270
Relative Vapor density (g/mL, air=1):Not available
Melting point (ºC): Not available
Boiling point (ºC, normal pressure): 105-107
Boiling point (ºC, 5.2kPa): Not available
Refraction Rate: 1.3893
Flash Point (ºC): 26
Optical rotation (º): Not available
Spontaneous combustion Point or ignition temperature (ºC): Not available
Steam Pressure (kPa, 25ºC): Not available
saturated Vapor pressure (kPa, 60ºC): Not available
Burn Heat (KJ/mol):Not available
Critical Temperature (ºC): Not available
Critical Pressure (KPa): Not available
oil and water Log value of the (octanol/water) partition coefficient:Not available
Explosion Upper limit (%, V/V): Not available
Explosion Lower limit (%, V/V): Not available
Dissolve Sex: Easily soluble in benzene, chloroform and other organic solvents, and soluble in water.
Toxicological data
Two , Toxicological data:
Acute Toxicity:Not available .
Ecological data
Three , Ecological data:
1 ,Other harmful effects: This substance may be harmful to the environment, and special treatment should be given to water bodies. Notice.
Molecular structure data
1. Molar refractive index: 25.72
2. Molar volume (m3/mol):121.7
3. isotonic specific volume (90.2K):264.8
4. Surface Tension (dyne/cm
Toxicological data
Two , Toxicological data:
Acute Toxicity:Not available .
Ecological data
Three , Ecological data:
1 ,Other harmful effects: This substance may be harmful to the environment, and special treatment should be given to water bodies. Notice.
Molecular structure data
1. Molar refractive index: 25.72
2. Molar volume (m3/mol):121.7
3. isotonic specific volume (90.2K):264.8
4. Surface Tension (dyne/cm):22.4
5. Polarizability(10-24cm3):10.19
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 5
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 159
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Should be sealed and stored in a cool place.
Synthesis method
None yet
Purpose
Solvent. Gas chromatography analysis separates chromium and rhodium. Extracting agent. Chelating agents. Identification, separation and purification of inorganic ions. Organic Synthesis. SPAN>
T-FAMILY: 宋体; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial; mso-bidi-font-family: Arial”>):22.4
5. Polarizability(10-24cm3):10.19
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 5
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 159
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Should be sealed and stored in a cool place.
Synthesis method
None yet
Purpose
Solvent. Gas chromatography analysis separates chromium and rhodium. Extracting agent. Chelating agents. Identification, separation and purification of inorganic ions. Organic Synthesis. SPAN>
