1,2,3-Trichlorobenzene
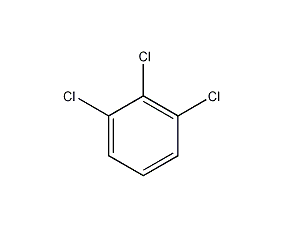

Structural formula
| Business number | 01Y7 |
|---|---|
| Molecular formula | C6H3Cl3 |
| Molecular weight | 181 |
| label |
None |
Numbering system
CAS number:87-61-6
MDL number:MFCD00000537
EINECS number:201-757-1
RTECS number:DC2095000
BRN number:956882
PubChem number:24862748
Physical property data
1. Properties: colorless liquid or plate crystal (in alcohol)
2. Relative density (g/mL, 100/4℃): 1.381
3. Relative density (solid): 1.69
4. Melting point (ºC): 53.5
5. Boiling point (ºC, normal pressure): 218.5
6. Refraction Rate (20ºC): 1.5776
7. Flash point (ºC): 126
8. Fire point (ºC): >500
9. Solubility: Insoluble in water, slightly soluble in ethanol, easily soluble in organic solvents such as ether, benzene, petroleum ether, carbon disulfide, chlorinated hydrocarbons.
10. Relative density (25℃, 4℃): 1.381298
11. Gas phase standard entropy (J·mol-1·K-1): 369.67
12. Gas phase standard hot melt (J·mol-1·K-1 ):128.71
Toxicological data
1. Acute toxicity
Rat caliber LD50: 1830mg/kg;
Mouse abdominal cavity LD50: 1390mg/kg;
2. Teratogenicity Sex
Mouse: 250mg/kg/24H;
3. Other multiple dose toxicity data
Rat caliber TDL0: 7020 mg/kg/13W- C;
4. Irritate eyes and respiratory tract, causing cough, sore throat and red eyes.
Ecological data
The substance is toxic to aquatic life.
Molecular structure data
1. Molar refractive index: 40.93
2. Molar volume (cm3/mol): 125.2
3. Isotonic specific volume (90.2K): 314.8
4. Surface tension (dyne/cm): 39.9
5. Polarizability (10-24cm3): 16.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 84.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Decomposes when heated or burned, producing toxic and corrosive fumes containing chlorine, hydrogen chloride and carbon monoxide. with�The oxidizing agent reacts.
Storage method
This product should be sealed and stored in a cool, dry place.
Synthesis method
1. The nontoxic body of 666 dried by pyrolysis method is heated in a pyrolysis kettle to obtain trichlorobenzene, and a large amount of hydrogen chloride is produced as a by-product. 2. Alkaline hydrolysis method: Trichlorobenzene is obtained by heating the nontoxic body of 666 with lime milk, and at the same time, a large amount of calcium chloride liquid is produced as a by-product. The trichlorobenzene obtained by the above two methods is a mixture of three isomers: 1,2,4-trichlorobenzene; 1,3,5-trichlorobenzene; 1,2,3-trichlorobenzene. , 4-trichlorobenzene is the main component. Among the trichlorobenzene produced by the lime milk method, the content of 1,2,4-trichlorobenzene is more than 75%, and 1,2,3-trichlorobenzene accounts for about 20%.
Purpose
Used as solvent and pharmaceutical intermediate. Used in the production of pesticides, dyes, transformer oil, electrolyte, lubricating oil and heat transfer media.
