1,3-di-o-tolyl-2-thiourea
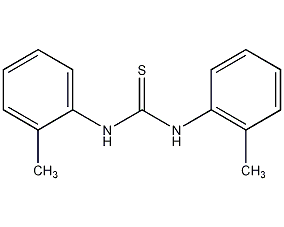
Structural formula
| Business number | 03RS |
|---|---|
| Molecular formula | C15H16N2S |
| Molecular weight | 256.37 |
| label |
N,N’-di-o-tolylthiourea, 2,2′-Xylenethiourea, Di(o-toluene)thiourea, N, N’-2-o-tolyl thiourea, 2,2′- xylene thiourea, 2 (o-Methylphenyl) thiourea, Thiourea accelerator |
Numbering system
CAS number:137-97-3
MDL number:MFCD00025922
EINECS number:205-309-6
RTECS number:FE0700000
BRN number:2125770
PubChem number:24848804
Physical property data
- Melting point (℃): 165~166
- Boiling point (sublimation, ℃): 216~218
- Characteristics: white needle-like crystals.
Toxicological data
None
Ecological data
None
Molecular structure data
Molecular property data:
1. Molar refractive index: 81.98
2. Molar volume (cm3/mol): 210.2
3. Isotonic specific volume (90.2K): 582.2
4. Surface tension (3.0 dyne/cm): 58.7
5. Polarizability (0.5 10-24cm3): 32.50
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.8
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 56.2
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 254
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Soluble in dimethylformamide, acetone, slightly soluble in carbon disulfide, benzene, chloroform, insoluble in xylene, carbon tetrachloride, ethanol and water. Can evaporate with water vapor.
Storage method
- Store in a cool, dry and ventilated place.
- Fire-proof, moisture-proof and sun-proof.
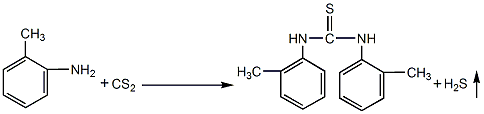
Synthesis methodLaw
- At room temperature, add 1% sulfur and 1% carbonic acid to the reaction solution composed of o-toluidine, water and a slight excess of carbon disulfide. Ammonium hydrogen or ammonia water is used as catalyst. Start stirring and perform the reaction under closed conditions. The initial pressure is 0.05~0.1MPa. When the pressure rises, the gas is released regularly, and the released hydrogen sulfide gas is absorbed by liquid alkali. The normal temperature reaction production cycle is long, about 15 days or more in summer.
- When the reaction temperature is controlled at about 40°C, the carbon disulfide is slightly excessive than the normal temperature method, and sulfur and ammonia are used as catalysts. The production cycle About 50h. When the reaction temperature is 70°C, the reaction time is 10h; when the reaction temperature is 80°C, the reaction time is 5h. At this time, the amount of carbon disulfide needs to be doubled, the amount of water is also increased, the catalyst is sulfur and sodium carbonate, and the carbon disulfide is added dropwise. A high pressure needs to be maintained during the reaction.
- After the condensation reaction is completed, filter, wash and dry to obtain the finished product.

Purpose
Uses:
- Used as boiler chemical cleaning corrosion inhibitor.
- This product is a thiourea vulcanization accelerator. Its function is very similar to the accelerator CA (diphenyl urea sulfur), but it is less likely to be scorched than the accelerator CA. It can be used together with thiazole vulcanization accelerator in natural rubber and 2-mercaptoimidazoline in chloroprene rubber to achieve rapid vulcanization.
