1,3-Diphenyltriazene

Structural formula
| Business number | 03QS |
|---|---|
| Molecular formula | C12H11N3 |
| Molecular weight | 197.24 |
| label |
phenylaminodiazobenzene, 1,3-diphenyl-1-triazene, diazoaminobenzene, Aniline azobenzene, benzene azoamine, Aniline diazobenzene, Diazoaminobenzene, foaming agent, polymerization initiator, accelerator for raw rubber |
Numbering system
CAS number:136-35-6
MDL number:MFCD00003021
EINECS number:Diazoaminobenzene
RTECS number:XY2625000
BRN number:None
PubChem number:24846930
Physical property data
1. Characteristics: Golden yellow shiny scaly crystals with a special odor.
2. Density (g/mL, 25/4℃): 1.17
3. Relative vapor density (g/mL, air=1): Undetermined 4. Melting point (ºC): 96~98
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 20Pa): 140
7. Refractive index: Undetermined8. Flash point (ºC): Undetermined 9. Decomposition temperature (ºC): 103℃ in air, 95~100℃ in plastic 10. Standard gas emission volume: 115ml/g11. Solubility: easily dissolved in natural rubber and neoprene rubber , soluble in ethanol and ether, insoluble in water.
Toxicological data
Oncogenic data:
Mouse oral TDLo: 1480mg/kg/59D-C
Mouse transdermal TDLo: 30mg/kg/46W-I
Mutation data:
Bacteria-Salmonella typhimurium: 300ng/plate
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 61.91
2. Molar volume (cm3/mol): 182.8
3. Isotonic specific volume (90.2K): 467.3
4. Surface tension (dyne/cm): 42.6
5. Polarizability (10-24cm3): 24.54
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 36.8
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 191
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain atoms Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Storage stable. Non-toxic. It will decompose at lower temperatures in acidic media, and the decomposition products are harmful to the human body. Production equipment should be sealed, and ventilation and exhaust should be strengthened at the production site. Operators should wear protective equipment.
Storage method
Should be sealed and stored in a ventilated, cool, dry place, and be protected from moisture and heat. It cannot be stored or transported together with acid or acidic substances.
Synthesis method
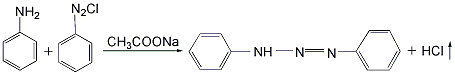
Put aniline and azobenzene chloride into the reactor at a mass ratio of 1:1.46, add water and stir to dissolve, then add an appropriate amount of sodium acetate as a catalyst, raise the temperature to 35°C, react with stirring for 1 hour, and then heat The temperature was raised to 65°C and reacted for 6 hours. The hydrogen chloride gas generated during the reaction is absorbed with water to obtain hydrochloric acid as a by-product. After the reaction solution is allowed to stand for 10 hours, sodium chloride is added to salt out to precipitate azoaminobenzene, and then is filtered and dried at low temperature to obtain the finished product.

Purpose
Complex titration indicator. Precipitating agent for palladium and copper. Determination of palladium and copper and separation from other ions. This product is used as a foaming agent for various rubbers and resins. Such as polyvinyl chloride and its copolymers, polystyrene, polyethylene, phenolic resin, epoxy resin, raw rubber and rubber, silicone polymers, etc. The dosage is 0.1%~5.0%. This product is also used as a polymerization initiator and accelerator for raw rubber.
