1,4-Benzenedimethanol
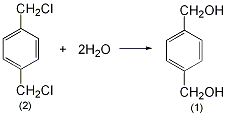
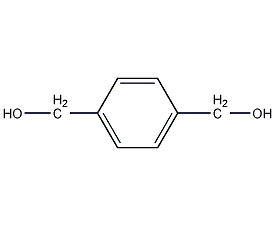
Structural formula
| Business number | 05ZZ |
|---|---|
| Molecular formula | C8H10O2 |
| Molecular weight | 138.17 |
| label |
terephthalol, p-Phenylenedimethanol, p-Xylene-α,α’-diol, p-Xylylene Glycol, C6H4(CH2OH)2 |
Numbering system
CAS number:589-29-7
MDL number:MFCD00004665
EINECS number:209-641-2
RTECS number:None
BRN number:2042077
PubChem number:24847533
Physical property data
1. Physical property data
1. Properties: White needle-like crystals.
2. Melting point (℃): 115-116
3. Boiling point (ºC, 133pa): 138-143
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 39.15
2. Molar volume (cm3/mol) 117.0
3. Isotonic specific volume (90.2K) : 314.3
4. Surface tension (dyne/cm): 51.9
5. Polarizability (10-24cm3): 15.52
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 73.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
1. Brief description of the production method
Obtained from the hydrolysis of p-phenylene dimethyl chloride. Add paraphenylene dimethyl chloride, sodium carbonate and water into the reactor, use industrial alcohol as the emulsifier, raise the temperature to below 90°C, stir and react for 3 hours to obtain the finished product. The yield is over 85%.
2. Preparation method:
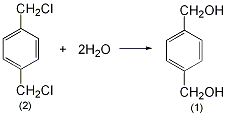
Add 1L of distilled water, 150g (0.52mol) of crystalline sodium carbonate, and 80g (0.46mol) of terephthalyl chloride (2) into the high-pressure reaction kettle, and heat to 150°C with stirring. When this temperature is reached, open the vent valve of the autoclave every 5 to 10 minutes to discharge the generated carbon dioxide. Keep the pressure in the autoclave at around 0.5MPa and around 150°C. After 3 hours, cool to 80°C, open the valve of the autoclave, pour out the reactants, and use a small amount ofWash the reaction kettle with water. The reaction solution was filtered while hot, then concentrated to about 200 mL and allowed to cool. Excess solid precipitated. Dissolve the solid in 250 mL of hot ethanol, filter out the insoluble inorganic salts, concentrate to about 130 mL, and cool to crystallize. After suction filtration and drying, 45g of terephthalenedimethanol (1) was obtained, mp was 115~117°C, and the yield was 75%. [1]
Purpose
2. Uses
Intermediates for organic synthesis. Used in the production of soluble polyphenylene.
