1,4-Difluorobenzene
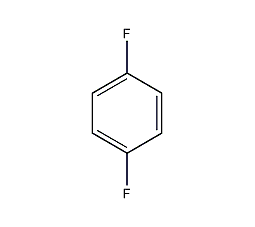

Structural formula
| Business number | 05K4 |
|---|---|
| Molecular formula | C6H4F2 |
| Molecular weight | 114.09 |
| label |
p-difluorobenzene, p-Difluorobenzene |
Numbering system
CAS number:540-36-3
MDL number:MFCD00000344
EINECS number:208-742-9
RTECS number:CZ5658000
BRN number:1904541
PubChem ID:None
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): -13[2]
3. Boiling point (℃): 88.8[3]
4. Relative density (water = 1): 1.17[4]
5. Octanol /Water partition coefficient: 2.13[5]
6. Flash point (℃): -5[6]
7. Solubility: Insoluble in water, soluble in ethanol, etc. [7]
8. Refractive index at room temperature (n20): 1.43422
9. Solubility parameter (J· cm-3)0.5: 18.588
10. van der Waals area (cm2·mol– 1): 6.820×109
11. van der Waals volume (cm3·mol-1): 55.520
12. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -342.42
13. Liquid phase Standard hot melt (J·mol-1·K-1): 134.9
14. Critical density (g·cm-3 ): 0.381
15. Critical volume (cm3·mol-1): 299
16 .Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -307.4
17. Gas phase standard entropy (J·mol-1 ·K-1): 315.55
18. Gas phase standard free energy of formation (kJ·mol-1): -252.4
19. Gas phase standard hot melt (J·mol-1·K-1): 106.46
Toxicological data
1. Acute toxicity[8] LC50: 55000mg/m3 (mouse inhalation, 2h )
2. Irritation No information available
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 26.24
2. Molar volume (cm3/mol): 97.8
3. Isotonic specific volume (90.2K ): 221.5
4. Surface tension (dyne/cm): 26.2
5. Polarizability (10-24cm3): 10.40
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 54.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine ��Number of stereocenters of chemical bonds: 0
14. Number of stereocenters of uncertain chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[9] Stable
2. Incompatible substances[10] Strong oxidizing agent
3. Polymerization hazard[11] No polymerization
4. Decomposition products [12] Hydrogen fluoride
Storage method
Storage Precautions[13] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Purpose
1. Used as an intermediate in organic synthesis. [14]
