1,4-octanolactone Octano-1,4-lactone
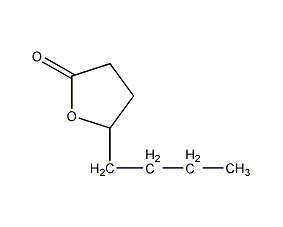

Structural formula
| Business number | 02Q5 |
|---|---|
| Molecular formula | C8H14O2 |
| Molecular weight | 142.20 |
| label |
4-octolactone, γ-Hydroxycaprylic acid lactone, γ-Octanoic lactone, 8-Oxo-5-octanolide, γ-Octalactone, 4-Octanolide |
Numbering system
CAS number:104-50-7
MDL number:MFCD00005402
EINECS number:203-208-1
RTECS number:LU3562000
BRN number:111677
PubChem number:24901331
Physical property data
1. Properties: colorless to slightly yellow oily liquid. It has a peach, coconut-like sweet fruit aroma and an oatmeal bread aroma.
2. Density (g/mL, 20℃): 0.981
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Not determined
5. Boiling point (ºC, normal pressure): 234, 132ºC (2666pa)
6. Boiling point (ºC, kPa): Not determined Confirm
7. Refractive index(n20D): 1.444
8. Flash Point (ºC): >110
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 20ºC): Undetermined
12. Saturation vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol ): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (polymer) Log value of the partition coefficient (alcohol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V ): Undetermined
19. Solubility: Soluble in ethanol and oil, slightly soluble in water, poorly soluble in propylene glycol.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 500mg/24HREACTION SEVERITY, moderate reaction; 2. Acute toxicity: rat oral LD50: 4400mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 38.77
2. Molar volume (cm3/mol): 146.8
3. Isotonic specific volume (90.2K ): 344.3
4. Surface tension (dyne/cm): 30.2
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.37
Scheduling�Chemical Data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 120
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides.
2. Found in burley tobacco leaves and oriental tobacco leaves.
3. Naturally found in apricots and peaches.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire, heat and water sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. It is made from the reaction of 1,2-epoxyhexane and sodium malonate, followed by saponification and lactonization.
2. Tobacco: BU, OR, 18.
Purpose
Suitable for heavy floral fragrances such as gardenia, tuberose, hyacinth, and even lily fragrances. It can also be used as a modifier or coumarin substitute in fragrant, lavender and herbal fragrances. It can also be used in coconut, nut, cream, vanilla bean, cheese caramel, rum and other food flavors.
