1,5-dihydroxyanthraquinone
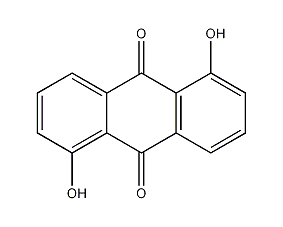

Structural formula
| Business number | 038Y |
|---|---|
| Molecular formula | C14H8O4 |
| Molecular weight | 240.21 |
| label |
Anthraquinones |
Numbering system
CAS number:117-12-4
MDL number:MFCD00001210
EINECS number:204-175-6
RTECS number:CB6630000
BRN number:1881718
PubChem ID:None
Physical property data
1. Appearance: yellow powder
2. Density (g/mL, 20℃): Undetermined
3. Relative vapor density (g/mL, air=1 ): Undetermined
4. Melting point (ºC): 280 (decomposition)
5. Boiling point (ºC, normal pressure): 280
6. Boiling point (ºC, KPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation Degree (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC) : Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Dissolved in Benzene and nitrobenzene are slightly soluble in ethanol, acetic acid and ether. They are red when soluble in potassium hydroxide solution. They are insoluble in sodium carbonate solution and ammonia. They are fluorescent red when soluble in concentrated sulfuric acid.
Toxicological data
1. Irritation: Rabbit eye: 500mg/24H Mild irritation.
Ecological data
Slightly harmful to water.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 62.43
2. Molar volume (cm3/mol): 155.9
3. Isotonic specific volume (90.2K): 465.2
4. Surface tension (dyne/cm): 79.2
5. Polarizability (10-24cm3): 24.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 15
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 342
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond establishment.Number of ��centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable at room temperature and pressure, avoid contact with strong oxidants.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and water sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
There are anthraquinone sulfonation method and benzoic acid sulfonation method. (1) Using anthraquinone as raw material and mercury sulfate as positioning agent, 1,5-anthraquinone disulfonic acid and 1,8-anthraquinone disulfonic acid are obtained through sulfonation. 1,5-Dihydroxyanthraquinone can be obtained by alkali melting and acidification of 1,5-anthraquinone disulfonic acid. (2) Using benzoic acid as raw material, it is obtained through sulfonation, alkali fusion, acidification, condensation and ring closure. This method does not use a mercury catalyst and eliminates mercury damage, but the one-step closed-loop yield is only 40% and consumes a large amount of aluminum trichloride. This method consumes more money, causes other pollution, and has high production costs.
Purpose
It is an intermediate for disperse dyes and acid dyes. Used in synthetic dyes.
