15-tetracosenoic acid
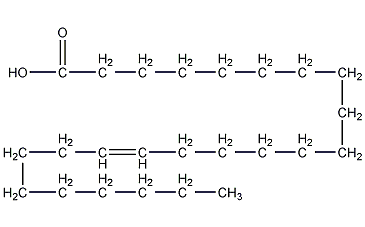

Structural formula
| Business number | 0599 |
|---|---|
| Molecular formula | C24H46O2 |
| Molecular weight | 366.62 |
| label |
nerve acid, cis-15-tetracosenoic acid, shark oil acid, cis-15-Tetracosenoic acid, Selacholeic acid, Nervonic acid, acidic solvent |
Numbering system
CAS number:506-37-6
MDL number:MFCD00010507
EINECS number:None
RTECS number:None
BRN number:1797907
PubChem number:24897496
Physical property data
1. Properties: light yellow oily liquid
2. Density (g/ cm3, 25/4℃): 0.887
3. Relative vapor density (g/cm3, air=1): Undetermined
4. Melting point (ºC): 42-43
5. Boiling point (ºC, normal pressure): 479.2
6. Boiling point (ºC, 8kPa): Undetermined
7. Refractive index: Undetermined
8. Flash Point (ºC): >110
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol ): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (polymer) Log value of the partition coefficient (alcohol/water): Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V ): Undetermined
19. Solubility: Undetermined
Toxicological data
None yet
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 114.85
2. Molar volume (cm3/mol): 412.9
3. Isotonic specific volume (90.2K ): 995.9
4. Surface tension (dyne/cm): 33.8
5. Polarizability (10-24cm3): 45.53
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 9.8
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors.��: 2
4. Number of rotatable chemical bonds: 21
5. Number of tautomers:
6. Topological molecule polar surface area ( TPSA): 37.3
7. Number of heavy atoms: 26
8. Surface charge: 0
9. Complexity: 309
10. The number of isotope atoms: 0
11. The number of determined atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 1
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Use and store according to specifications. It will not decompose and avoid contact with oxides.
2. Exist in smoke.
Storage method
Seal and store in a ventilated, dry environment
Synthesis method
Nervonic acid was first discovered in mammalian nervous tissue. It is an important component of biofilms in nervous tissue and a signature component of the medulla in cerebral glycosides. Currently mainly derived from shark brain and shark oil, it is a new generation of health care additives that is extremely popular at home and abroad.
Purpose
Nervonic acid is recognized by scientists from all over the world as the world’s first and only dual-effect miraculous substance that can repair and unblock damaged nerve pathways in the brain – nerve fibers, and promote the regeneration of nerve cells.
Nervonic acid is the core natural component of brain nerve fibers and nerve cells. The lack of nervonic acid will cause brain diseases such as stroke sequelae, Alzheimer’s disease, cerebral palsy, brain atrophy, memory loss, insomnia and forgetfulness. Nervous acid is difficult to produce by the human body itself, so it can only be supplemented by ingestion outside the body
Nervous acid can completely penetrate the blood-brain barrier, directly act on nerve fibers to repair and dredge, and regenerate damaged and fallen protective sheaths. Dissolve the necrotic tissue blocking the channels, induce the self-growth and division of nerve fibers, so that the information generated by nerve cells and external information can be smoothly transmitted through nerve fibers to achieve smooth flow of instructions, thereby activating damaged, diseased and dormant nerves Cells, reshape neural networks, restore part or all of the patient’s functions in language, memory, sensation, limbs, etc., to achieve complete recovery from encephalopathy.
