2-Ethyl-1,3-hexanediol 2-Ethyl-1,3-hexanediol
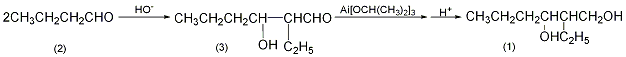
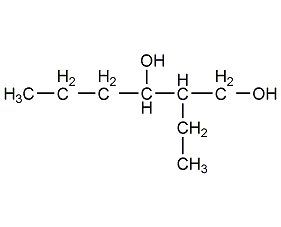
Structural formula
| Business number | 028E |
|---|---|
| Molecular formula | C8H18O2 |
| Molecular weight | 146.23 |
| label |
2-ethylhexane-1,3-diol, repellent, 2-ethyl-1,3-hexanediol, 2-Ethyl-3-hexanediol, 2-Ethyl-1,3-hexylene glycol, 2-Ethyl-3-propyl-1,3-propanediol, Octylene glycol, Ethohexadiol, CH3CH2CH2CH(OH)CH(C2H5)CH2OH, Mosquito and fly repellent, ink solvent, alcohol solvents, Cosmetic raw materials |
Numbering system
CAS number:94-96-2
MDL number:MFCD00004578
EINECS number:202-377-9
RTECS number:MO2625000
BRN number:1735324
PubChem number:24894489
Physical property data
1. Properties: Colorless, odorless and slightly viscous liquid.
2. Relative density (g/mL, 20/4℃): 0.9405
3. Relative vapor density (g/mL, air=1): 5
4. Melting point (ºC): -40
5. Boiling point (ºC, 101.3kPa): 243.2
6. Refractive index (20ºC): 1.4511
7. Flash point (ºC, open): 127
8. Viscosity (mPa·s, 20ºC): 323
9. Solubility (%, 20ºC, water) : 4.2
10. Vapor pressure (kPa, 20ºC): <0.0013
11. Heat of evaporation (KJ/kg): 395.2
12. Solubility : Slightly soluble in water, soluble in ethanol and ether.
Toxicological data
1. Acute toxicity: Rat LD50: 1400mg/Kg; mouse mouth LD50: 1900mg/kg
Rat via leather LD50: 2000mg/kg
2. Irritation data: Skin – 500 mg for rabbits, mild; Eyes – 20 mg for rabbits, severe
3. It is of low toxicity. The vapor pressure is low at room temperature, so poisoning by inhaling the vapor is unlikely. Inhalation of large doses causes deep anesthesia. It is also less irritating to the skin and mucous membranes and is generally regarded as a harmless drug. Guinea pig oral LD50: 4.2mL/kg.
Ecological data
Slightly harmful to water.
Molecular structure data
1.Molar refractive index: 42.09
2. Molar volume (cm3/mol): 156.3
3. Isotonic specific volume (90.2K): 378.6
4. Surface tension (dyne/cm): 34.3
5. Dielectric constant: not useful
6. Dipole moment (10-24cm3): No use
7. Polarizability: 16.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.3
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 73.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, strong reducing agents, strong acids, acid chlorides, and acid anhydrides. Flammable liquid, non-corrosive to metals. Contains asymmetric carbon atoms, has optical isomers, and also has the chemical properties of general glycols.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, reducing agents, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. Can be stored in iron, mild steel, copper or aluminum containers.
Synthesis method
1. First, butyraldehyde is synthesized from propylene. Under the catalysis of alkali or acid, butyraldehyde condenses itself to form 2-ethyl-3-hydroxyhexanal. This compound is very unstable, and upon hydrogenation, 2-ethyl-1,3-hexanediol is obtained.
2. Preparation method:
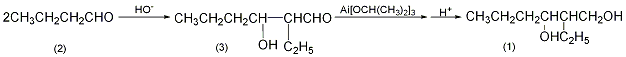
2-Ethyl-3-hydroxyhexanal (3): In a reaction bottle equipped with a stirrer, thermometer, and dropping funnel, add 260g (3.6mol0) of n-hexanal (3) and 150mL of diethyl ether. Cool to -3°C in the ice-salt bath, and begin to add 30 mL (10%) of potassium aldehyde oxide solution dropwise. The dripping should be slow at the beginning to prevent the temperature from exceeding 8°C. It takes about 3 hours to complete. After the addition was completed, the reaction was stirred for 5 hours. The organic layer was separated, washed three times with water, and dried over anhydrous sodium sulfate. The diethyl ether was recovered, and the low boiling matter was evaporated under reduced pressure to obtain 200 to 220 g of crude product 2-ethyl-3-hydroxyhexanal (3). 2-Ethyl-1,3-hexanediol (1): In a reaction bottle equipped with a stirrer and a reflux condenser, add 33g (1.2mol) aluminum chips, 300mL anhydrous isopropyl alcohol, and add 0.1g iodine , heating in a water bath, generates aluminum isopropoxide, and releases a large amount of hydrogen. When most of the aluminum participates in the reaction, add 300 mL of anhydrous isopropyl alcohol. Reflux for 6h to complete the reaction. The reaction system is dark gray. After cooling, add the 2-ethyl-3-hydroxyhexanal (3) prepared previously, install a packed fractionating column, and continuously evaporate the acetone generated. As the reaction proceeds, acetone gradually evaporates and isopropanol evaporates until the temperature at the top of the column reaches 80°C (at this time it is isopropanol and can be recycled and reused). Cool and adjust to weak acidity with 15% sulfuric acid, and oily matter will precipitate. Separate the oil, wash it with water three times, dry it over anhydrous sodium sulfate, and then perform fractional distillation. Collect the fractions at 238-242°C to obtain 60g of 2-ethyl-1,3-hexanediol (1), with a yield of 23%. [1]
Purpose
Used in organic synthesis to produce polyester resin, polyester plasticizer, polyurethane resin, etc. This product is an effective insect repellent against mosquitoes and flies, and can also be used in the production of cosmetics or as an ink solvent. Used as boric acid complexing agent, medicine, vehicle and solvent for coatings, cosmetics, insect repellent, etc.
