2-Ethylhexyl acrylate 2-Ethylhexyl acrylate
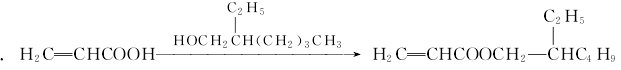
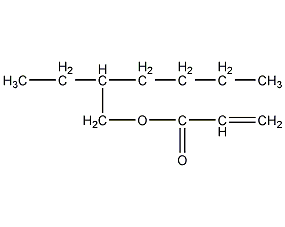
Structural formula
| Business number | 02MX |
|---|---|
| Molecular formula | C11H20O2 |
| Molecular weight | 184.28 |
| label |
2-ethylhexyl acrylate, 2-ethylhexyl acrylate, Isooctyl acrylate, 2-Propenoic acid, 2-ethylhexyl ester, Acrylic acid 2-ethy-hexyl ester, Multifunctional solvents, aliphatic compounds |
Numbering system
CAS number:103-11-7
MDL number:MFCD00009495
EINECS number:203-080-7
RTECS number:AT0855000
BRN number:1765828
PubChem number:24885123
Physical property data
1. Properties: Colorless and transparent liquid, odorless and tasteless.
2. Density (g/mL, 20/4℃): 0.8845
3. Relative vapor density (g/mL, air=1): 6.35
4. Melting point (ºC): -90
5. Boiling point (ºC): 123~127 (7998pa)
6. Refractive index (n20ºC): 1.4365
7. Viscosity (mPa·s, 20ºC): 1.54
8. Flash point (open cup, ºC): 86
9. Autoignition point Or ignition temperature (ºC): 252
10. Vapor pressure (mmHg, 20ºC): 0.15
11. Vapor pressure (kPa, 20ºC): 0.02
12. Heat of vaporization (KJ/mol): 47.0
13. Explosion upper limit (%, V/V): 6.4
14. Explosion lower limit (%, V/V ): 0.9
15. Solubility: Miscible with ethanol and ether, slightly soluble in water.
16. Liquid phase standard hot melt (J·mol-1·K-1): 380.8
Toxicological data
1. Irritation: Rabbit transdermal: 20mg/24 hours, moderate irritation. Rabbit transdermal: open stimulation test, 500mg, mild stimulation. Rabbit eye: 5mg, severe irritation. Rabbit eye: 500mg/24 hours, mild irritation.
2. Acute toxicity: rat oral LD50: 5600mg/kg; rabbit transdermal LD50: 7539mg/kg
3. Irritating to eyes, respiratory system and skin.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 54.46
2. Molar volume (cm3/mol): 209.0
3. Isotonic specific volume (90.2K ): 480.9
4. Surface tension (dyne/cm): 27.9
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. �Transformation rate: 21.59
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 152
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Light is easy to aggregate. Avoid contact with strong oxidants, strong acids, and strong alkali. It is flammable and can burn when exposed to open flame or high heat.
2. Exist in mainstream smoke.
3. Sensitive to light, tear-inducing.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep away from light. The storage temperature should not exceed 30℃. Keep container sealed and strictly prohibited from contact with air. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. Storage temperature 2-8ºC
Synthesis method
1. Direct esterification method: Acrylic acid and 2-ethylhexanol are esterified using sulfuric acid as a catalyst, and then neutralized, dealcoholized and distilled to obtain the finished product.
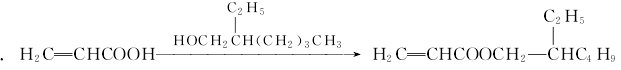
2. Transesterification method of acrylic acid The ester and 2-ethylhexanol undergo a transesterification reaction in the presence of the catalyst titanium tetrachloride to generate 2-ethylhexyl acrylate, which is then distilled to obtain the finished product.
3.Mix methyl acrylate and 2-ethylethanolamine at 3:1 (molar ratio), and conduct gas phase at 227~228℃ Transesterification reaction, the catalyst is titanium tetrachloride supported on silica gel, the conversion rate is 32%, the yield is 97%, when the transesterification reaction is carried out under liquid phase conditions, the yield is 70%~80%
4.Add a certain amount of acrylic acid to the reaction kettle , isooctyl alcohol, catalyst (SO42-/ZrO2) and polymerization inhibitor phenothiazine, heat and reflux until no water comes out. The reaction solution is washed with alkali and water until neutral and distilled under reduced pressure to collect the product. The catalyst dosage is 5% of the mass of acrylic acid, the polymerization inhibitor dosage is 0.05% of the mass of acrylic acid, the raw material ratio is (isooctanol): (acrylic acid) = 1.2:1, the reaction temperature is 120°C, and the reaction time is 3 hours.
Purpose
1. Used in the manufacture of coatings, adhesives, fiber and fabric modifications, processing aids, leather processing aids, etc. It is used as a polymerization monomer for soft polymers and plays an internal plasticizing role in copolymers. Also used as solvent.
2.Mainly used as a soft monomer in the manufacture of acrylic solvent-based and emulsion-based pressure-sensitive adhesives. It is also used as the main monomer for the production of microsphere pressure-sensitive adhesive for notebooks. It is also used in the manufacture of coatings, plastic modifiers, paper and leather processing aids, fabric finishing agents and other products.
3. Used in the processing of synthetic fiber fabrics and as adhesive (anti-invasive pressure-sensitive adhesive).
