2-Ethylhexyl vinyl ether 2-Ethylhexyl Vinyl Ether
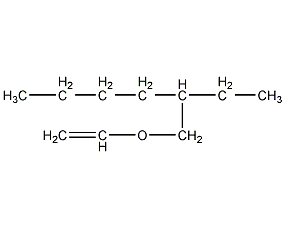

Structural formula
| Business number | 02NK |
|---|---|
| Molecular formula | C10H20O |
| Molecular weight | 156.27 |
| label |
Octyl vinyl ether, Isooctyl Vinyl Ether |
Numbering system
CAS number:103-44-6
MDL number:MFCD00053800
EINECS number:203-111-4
RTECS number:KO0175000
BRN number:None
PubChem number:24865679
Physical property data
1. Properties: liquid.
2. Density (g/mL, 20℃): 0.810
3. Relative vapor density (g/mL, air=1): 5.4
4. Melting point (ºC): -100
5. Boiling point (ºC, normal pressure): 7632
6. Relative density (20℃, 4℃): 0.8108
7. Refractive index: 1.428
8. Flash point (ºC): 47
9. Refractive index at room temperature (n25): 1.4247
10. Autoignition point or ignition temperature (ºC): 202
11. Vapor pressure (mmHg, 20.2ºC): Undetermined
12. Saturated vapor pressure (kPa, 20ºC): 0.123
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC ): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Slightly Dissolved in water.
Toxicological data
1. Skin/eye irritation: Start irritation test: rabbit skin contact, 500mgREACTION SEVERITY, slight reaction; 2. Acute toxicity: rat oral LD50: 1350mg/kg; rat inhalation LCLo: 1005ppm/8H; rabbit skin contact LD50 :3560μL/kg;
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 49.80
2. Molar volume (cm3/mol): 195.4
3. Isotonic specific volume (90.2K ): 436.0
4. Surface tension (dyne/cm): 24.7
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 19.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 7
5. MutavariationNumber of solids: None
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 88.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain stereocenters of atoms: 1
13. The number of determined stereocenters of chemical bonds: 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants, oxygen, and acids.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container sealed and strictly prohibited from contact with air. They should be stored separately from oxidants and acids, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
None yet
Purpose
Used in organic synthesis.
