2-Isopropoxyethanol 2-Isopropoxyethanol
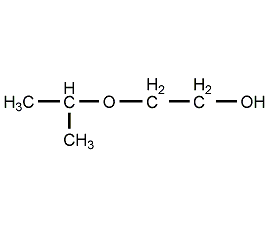

Structural formula
| Business number | 02Z8 |
|---|---|
| Molecular formula | C5H12O2 |
| Molecular weight | 104.15 |
| label |
Ethylene glycol monoisopropyl ether, 2-Isopropoxyethanol, Ethylene glycol isopropyl ether, Isopropoxyethanol, Ethylene glycol isopropyl ether, 2-isopropoxyethanol/ethylene glycol monoisopropyl ether, 2-(1-Methylethoxy)-ethanol, 2-Isopropoxy-ethanol, beta-Hydroxyethyl isopropyl ether, Ethylene glycol isopropyl ether, solvents for coatings, aliphatic compounds |
Numbering system
CAS number:109-59-1
MDL number:MFCD00002866
EINECS number:203-685-6
RTECS number:KL5075000
BRN number:1732184
PubChem number:24846786
Physical property data
1. Properties: colorless liquid with slightly unpleasant odor.
2. Density (g/mL, 20/20℃): 0.906
3. Relative vapor density (g/mL, air=1): 3.6
4. Boiling point (ºC, normal pressure): 139~143
5. Refractive index (26ºC): 1.4048
6. Flash point (ºC, open): 33
7. Vapor pressure (kPa, 20ºC): 0.80
8. Volume expansion coefficient (K-1, 10~30ºC): 0.00093
9. Solubility: Miscible with water and various organic solvents.
Toxicological data
1. Irritation: Rabbit transdermal: 20mg/24 hours, moderate irritation. Rabbit eye: 500mg/24 hours, moderate irritation.
2. Acute toxicity: LD50: 500~1000 mg/kg (oral in rats); 4900 mg/kg (oral in mice) LC50: 3100mg/m3, 4 hours (inhalation in rats) ; 1930mg/m3, 7 hours (mouse inhalation)
3. Serious damage to kidneys and liver. Instillation into rabbit eyes can cause significant conjunctival irritation and corneal damage. Prolonged contact with skin may cause significant irritation or even burns. A lethal dose is rapidly absorbed through the skin.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 58.85
2, Molar volume (cm3/mol): 178.1
3, Isotonic specific volume (90.2K): 447.5
4. Surface tension (dyne/cm): 39.8
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 23.33
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 35.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidants and acids. Ethylene glycol-isopropyl ether is non-corrosive to metals.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Refining method: dry with calcium chloride or anhydrous sodium sulfate and then fractionate.
Purpose
Used as a solvent for coatings such as nitrocellulose paint. Due to its low hygroscopicity, when used as a coating solvent, the coating film is not prone to emulsification.
