2-Methoxy-5-methylaniline 2-Methoxy-5-methylaniline
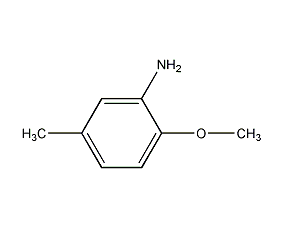
Structural formula
| Business number | 03CP |
|---|---|
| Molecular formula | C8H11NO |
| Molecular weight | 137.18 |
| label |
2-amino-4-methylanisole, p-cresolidin, Methoxycrine, christine, Crichidine, o-Chloro-p-methylanisole, 2-methoxy-5-methylaniline, 3-Methyl-6-methoxyaniline, 2-Amino-4-methylphenolmethylether, 2-Amino-p-cresol methyl ether, 2-Amino-p-cresolmethylether, 2-methoxy-5-methyl-benzenamin, 2-Methoxy-5-methylbenzenamine, 2-methoxy-5-methyl-Benzenamine, 3-amino-para-cresol,methylether, 4-Methyl-2-aminoanisole, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:120-71-8
MDL number:MFCD00007815
EINECS number:204-419-1
RTECS number:BZ6720000
BRN number:637071
PubChem number:24869810
Physical property data
1. Appearance: white crystal
2. Melting point (℃): 51.5
3. Boiling point (ºC): 235
4. Solubility : Insoluble in water, soluble in ethanol, ether and hydrochloric acid, insoluble in benzene.
Toxicological data
Toxic, rat oral LD50: 1450mg/kg.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 41.99
2. Molar volume (cm3/mol): 131.9
3. Isotonic specific volume (90.2K): 327.4
4. Surface tension (dyne/cm): 37.8
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability 16.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 35.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 105
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain atomic stereocenterNumber of stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
This product is toxic and irritating. Emit toxic gases when heated. In case of skin contact, rinse with water and wash thoroughly with soap.
Storage method
The packaging is made of glass bottles with wooden boxes lined with padding. Store in a cool, ventilated warehouse, away from fire and heat sourcesavoid direct sunlight, and isolated from edible raw materials for storage and transportation.
Synthesis method
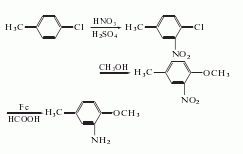
1. Obtained from nitration, methoxylation and reduction of p-chlorotoluene. Add the mixed acid to p-chlorotoluene at 15-20°C, stir for 2 hours at 30°C, dilute with water, separate the spent acid, and wash with water to obtain 4-chloro-3-nitrotoluene (yield 30.6%) and 4-chloro-2- Nitrotoluene (yield 56.7%), separate the two by fractionation and crystallization to obtain 4-chloro-3-nitrotoluene. React it with methanol and sodium methoxide solution, neutralize and separate with sulfuric acid to obtain 3-nitro-4-methoxytoluene, with a yield of 85.6%. After reduction with water, iron powder and formic acid, it is obtained by multiple extraction and distillation with solvent gasoline.
2. Nitration of p-cresol 2-nitro-4-cresol is obtained, which is then methylated with dimethyl sulfate to 3-nitro-4-p-methoxytoluene, and finally reduced with alkali sulfide. 3. p-Chloro-N,N-diethylbenzylamine method: obtained through nitrification, methoxylation and reduction reactions.

Purpose
This product is a dye intermediate. In terms of direct dyes, it is used to synthesize C.I.29050, 29065, 27885, etc. In terms of acid dyes, it is used to synthesize C.I.14940, 14965, etc. It is also used to produce dispersed red GC, direct light fast blue 3RLL, direct black D, and reactive yellow brown K. -GR, cationic yellow 4G, etc.
