2-methylquinoline
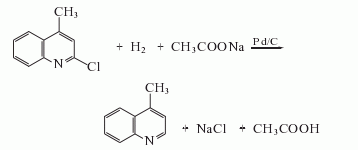
Structural formula
| Business number | 023U |
|---|---|
| Molecular formula | C10H9N |
| Molecular weight | 143 |
| label |
o-Methylquinoline, quinaldine, Quinaldine, o-Methylquinoline, Heterocyclic compounds |
Numbering system
CAS number:91-63-4
MDL number:MFCD00006756
EINECS number:202-085-1
RTECS number:UZ9625000
BRN number:110309
PubChem number:24899328
Physical property data
1. Properties: colorless oily liquid. Has a quinoline smell. Sensitive to light. It turns reddish-brown when exposed to air.
2. Density (g/mL, 25/4℃): 1.06
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): Not determined
5. Boiling point (ºC, normal pressure): 246~247
6. Boiling point (ºC, 5.2kPa): Not determined Determined
7. Refractive index: 1.6116
8. Flash point (ºC): 79
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether and chloroform, almost insoluble in water
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 45.95
2. Molar volume (cm3/mol): 145.7
3. Isotonic specific volume (90.2K ): 365.3
4. Surface tension (dyne/cm): 39.4
5. Polarizability (10-24cm3): 18.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 12.9
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
It can be extracted from coal tar or prepared synthetically. The sulfate quinoline salt base solution obtained by washing the naphthalene oil fraction and the washed oil fraction with sulfuric acid is steamed to remove impurities such as neutral oil, and then decomposed with alkali or ammonia. The separated crude quinoline and base homologues are dehydrated. Finally, use a high-efficiency distillation tower with 60 theoretical plates for rectification, and cut the fraction section with a boiling range of 237.5-239.5°C in the base to obtain the crude quinoline; continue to cut the fraction section with a boiling range of 243-246°C as Extract the raw materials of isoquinoline and 2-methylquinoline; continue to cut the fraction section at 260-267°C as the raw material for extracting 4-methylquinoline. 2-Methylquinoline is isolated in the form of hydrochloride from the fraction at 243-246°C. The 2-methylquinoline hydrochloride crystal is washed with benzene, and then the salt is decomposed with 20% sodium hydroxide solution. 246.6 are cut by distillation. -247.4°C fraction, 2-methylquinoline with a purity greater than 95% can be obtained. 2-Methylquinoline can be synthesized by reacting aniline with 2,3-dibromobutyraldehyde.
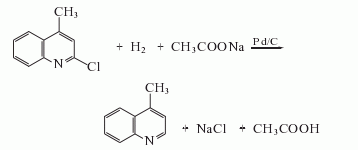
Purpose
Metal ion precipitating agent. Organic synthesis intermediates. Determination of bismuth.
