2,2-Bis(4-hydroxyphenyl)butane 2,2-Bis(4-hydroxyphenyl)butane
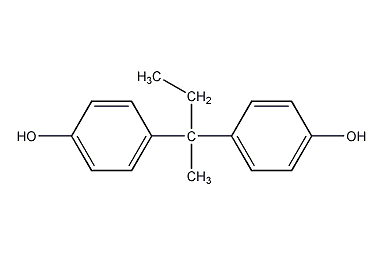

Structural formula
| Business number | 01LR |
|---|---|
| Molecular formula | C16H18O2 |
| Molecular weight | 242.31 |
| label |
Bisphenol B, 2,2-bis(4-hydroxyphenyl)butane, p,p’-sec-Butylidenediphenol, 2,2-Bis(p-hydroxyphenyl)butane, Bis(4-hydroxyphenyl)methylethylmethane, p,p’-Dihydroxy-2,2-diphenylbutane |
Numbering system
CAS number:77-40-7
MDL number:None
EINECS number:201-025-1
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
1. Characteristics: white crystal.
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC):: 124℃
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index: Uncertain
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
12. ):559.5
4. Surface Tension (dyne/cm):44.9
5. Polarizability(10-24cm3): 28.86
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 3
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 226
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
this The product should be sealed and stored in a cool place.
Synthesis method
Experiment In-house preparation method: 100 mL In a three-necked flask,Add the measured amount of phenol, Main catalyst concentrated hydrochloric acid, Heat to set temperature A certain temperature,Add measured Ding Ketones and cocatalysts, Constant temperature stirring reaction is certain Time. Pour while hot100mL Cooling in ice water,Leave overnight. Reduced pressure filtration,Wash in cold water until neutral. Vacuum drying of filter cake to remove moisture and high vacuum to remove phenol,The solid was recrystallized from toluene,Very pure bisphenol can be obtainedB.
Purpose
Chemical Engineering intermediate. Generally used in petroleum, energy and chemical industries.
amily: ‘Times New Roman'”>Constant temperature stirring reaction for a certain time. Pour while hot100mL Cool in ice water,Let it sit overnight . Decompression filter,Wash in cold water until neutral. Vacuum drying of the filter cake to remove moisture and high vacuum to remove phenol,The solid was recrystallized from toluene,Very pure bisphenol can be obtainedB.
Purpose
Chemical Engineering intermediate. Generally used in petroleum, energy and chemical industries.
