2,4-Dichlorophenoxybutyric acid 2,4-DB Acid
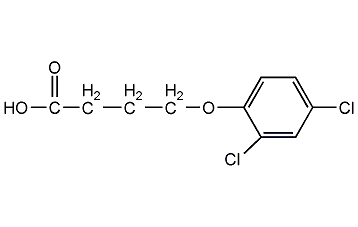

Structural formula
| Business number | 0288 |
|---|---|
| Molecular formula | C10H10Cl2O3 |
| Molecular weight | 249.09 |
| label |
2,4-D butyric acid, 2,4-D butyric acid, 4-(2,4-Dichlorophenoxy)butyric acid, Butyrac,Legumex,Embutox, herbicide |
Numbering system
CAS number:94-82-6
MDL number:MFCD00002819
EINECS number:202-366-9
RTECS number:ES9100000
BRN number:1976809
PubChem number:24855841
Physical property data
1. Properties: white crystal.
2. Density (g/mL, 20℃): 1.2428
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 117~119
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, KPa): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC): Undetermined
12. Saturated vapor pressure ( kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/ V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Hardly soluble in water, easily soluble in most organic solvents.
Toxicological data
1. Acute toxicity: rat oral LD50: 700mg/kg; rat skin contact LD50: 800mg/kg; mouse oral LDLo: 750mg/kg; rabbit skin contact LD50: >10mg/kg;
2. Other multiple dose toxicity: Rat oral TDLo: 18928mg/kg/13W-I; Rat oral TDLo: 4368mg/kg/60W-I; Rat oral TDLo: 5mg/ kg/10D-I;
3. Reproductive toxicity
Rat oral TDLo: 17mg/kg (female rats are pregnant for 1-7 days); Rat oral TDLo: 416mg /kg (5 days after conception in female rats); Rat oral TDLo: 416 mg/kg (9 days after conception in female rats);
4. Mutagenicity
E. coli DNA repair :5mg/disc;
Ecological data
This substance is harmful to the environment and it is recommended not to let it enter the environment. Special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 58.18
2. Molar volume (cm3/mol): 181.4
3.Isotonic specific volume (90.2K): 476.8
4. Surface tension (dyne/cm): 47.6
5. Polarizability (10-24cm3): 23.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 46.5
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 211
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. The packaging is sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Synthesis of 2,4-D: Sodium phenolate and sodium chloroacetate are first synthesized, and then the two are condensed. The reaction is carried out at 110-120°C. The reaction process maintains the slightly alkaline solution. The feeding ratio of monochloroacetic acid to phenol is 1:1.16. Then a chlorination reaction is carried out, using chlorine gas as the chlorinating agent, and the reaction temperature is 92 to 98°C.
It has also been reported that the following operation method is used: After mixing 31.6g (0.332mol) of industrial phenol and an appropriate amount of toluene, the temperature is raised to near the reflux point, and aqueous solutions of sodium hydroxide and chloroacetic acid are added dropwise at the same time. The dropping time is 1 hour, and the reaction is carried out at the reflux temperature. After 1 hour, add 230 mL of tap water to the reaction, separate the organic phase solvent and recycle it to obtain phenoxyacetic acid, which can be directly used for chlorination reaction without distillation operation. Use chlorine gas as the chlorinating agent. Add a trace amount of catalyst (such as iodine powder), the chlorination time is 1 to 3.5 hours, the chlorination temperature is 65 to 90°C, the chlorination is completed by filtering and washing at room temperature, and drying to obtain 57.0g of 2,4-D. The total yield of the two steps is ≥ 76%, the quality is significantly improved.
Synthesis of 2,4-D butyl ester Add wet 2,4-D to a certain amount of butanol under stirring, raise the temperature to 120~140°C, keep it for 4 hours, fully dehydrate, and reflux the butanol. When the water level in the separator no longer rises, stop the reflux, raise the temperature to 160-170°C, and steam out the butanol under reduced pressure.
Purpose
Used as agricultural herbicide.
