2,4-Dihydroxybenzophenone
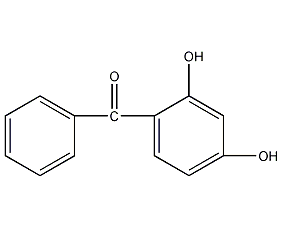
Structural formula
| Business number | 03N2 |
|---|---|
| Molecular formula | C13H10O3 |
| Molecular weight | 214.22 |
| label |
(2,4-Dihydroxyphenyl)phenylketone, 2,4-Dihydroxybenzophenone, 2,4-Dihydroxybenzophenone (UV-O), 2,4-dihydroxybenzophenone, UV-O ultraviolet absorber, Ultraviolet Absorbent UV-0, Ultraviolet Absorbent UV-214, UV absorber |
Numbering system
CAS number:131-56-6
MDL number:MFCD00002277
EINECS number:205-029-4
RTECS number:DJ0700000
BRN number:1311566
PubChem number:24863420
Physical property data
1. Properties: light yellow needle-like crystals or white powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 136~149
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in acetone, methanol, ethanol, Methyl ketone, dioxane, N-methylpyrrolidone and ethyl acetate are extremely difficult to dissolve in water, n-heptane and benzene.
20. Maximum absorption peak: 322nm
Toxicological data
1. Skin/eye irritation toxicity: Rabbit eye standard Drez eye dye test: 100mg/24H has a moderate irritating effect on the eyes.
2. Acute toxicity: Rat oral LD5O: 8600mg/kg
Mouse intraperitoneal LC5O: 100mg/kg
Mouse intravenous LC5O: 85mg/kg
3. Other multiple dose toxicity: Rat oral TDLO: 54600mg/kg/91D-I
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 59.80
2. Molar volume (cm3/mol): 164.4
3. Isotonic specific volume (90.2K ): 456.9
4. Surface tension (dyne/cm): 59.6
5. Polarizability (10-24cm3): 23.71
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 12
6. Topological molecule polar surface area 57.5
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 246
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
UV absorber, non-flammable, non-explosive, non-corrosive, and has good storage stability.
Storage method
Non-flammable, non-explosive, non-corrosive, and has good storage stability. Packed in cardboard drums lined with plastic bags.
Synthesis method
There are 4 main production methods: 1. Use resorcinol and benzoyl chloride as raw materials. Add benzoyl chloride and resorcinol to the reactor, then add the solvent chlorobenzene and the catalyst aluminum trichloride for reaction to remove hydrogen chloride. , generating 2,4-dihydroxybenzophenone. After the reaction is completed, the reaction product is distilled to remove the solvent and low boiling matter, and then decolorized and dried to obtain the finished product. The product of this method has good color, almost white crystal, but the cost of raw materials is high, and the reaction yield is only 50% to 60%. %, and produce a large amount of waste catalyst. 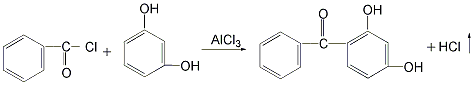 2. Use resorcinol and trichlorotoluene as raw materials in the reaction kettle Add resorcinol and appropriate amount of water and stir to dissolve. Then add trichlorotoluene and an appropriate amount of alcohol, and stir to make the materials completely miscible. The reaction was carried out at 40°C, and 2,4-dihydroxybenzophenone was generated and precipitated as a solid. After the reaction is completed, the reactants are filtered, and the filter cake is washed with dilute sodium bicarbonate solution and then dried to obtain the finished product. The yield of this method can reach 95%, the raw materials are easy to obtain, and the product cost is low. However, the product has a darker color and is not easy to decolorize and purify.
2. Use resorcinol and trichlorotoluene as raw materials in the reaction kettle Add resorcinol and appropriate amount of water and stir to dissolve. Then add trichlorotoluene and an appropriate amount of alcohol, and stir to make the materials completely miscible. The reaction was carried out at 40°C, and 2,4-dihydroxybenzophenone was generated and precipitated as a solid. After the reaction is completed, the reactants are filtered, and the filter cake is washed with dilute sodium bicarbonate solution and then dried to obtain the finished product. The yield of this method can reach 95%, the raw materials are easy to obtain, and the product cost is low. However, the product has a darker color and is not easy to decolorize and purify. 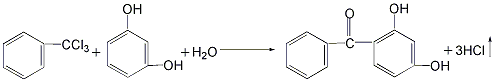
3. Using benzoic acid and resorcinol as raw materials, combine benzoic acid and resorcinol The diphenol and the catalyst zinc oxide are put into the reaction kettle, and the reaction is carried out by heating under stirring. Phosphorus trichloride or phosphoric acid is added to the reaction to increase the dehydration reaction speed.
The yield of this method can reach more than 90%. However, the raw material benzoic acid is easy to sublime and adhere to the reactor wall. The reaction time is long and the melt discharge operation is difficult.
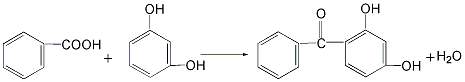
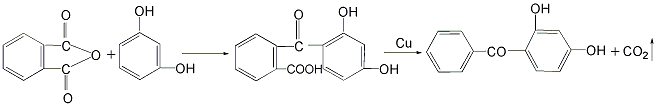
4. Combine phthalic anhydride and isophthalic anhydride Diphenol is added to the reaction kettle, and then solvent quinoline and catalyst copper powder are added to perform a condensation reaction to obtain 2-(2′,4′-dihydroxybenzoyl)benzoic acid, which is then decarboxylated to obtain 2,4-dihydroxybenzoic acid. Hydroxybenzophenone.
This method has a low yield.

Purpose
1. This product is a UV absorber, has good compatibility with most synthetic resins, and is widely used in polymer materials. This product is suitable for polyvinyl chloride, cellulose resin, unsaturated resin, coatings and synthetic rubber, etc., with a maximum absorption wavelength range of 280~340nm. The general dosage is 0.1% to 1%. The light stabilizing effect of this product is not outstanding.
2.UV absorber. Mainly used in sunscreen and tanning cosmetics. It can absorb ultraviolet light with a wavelength range of 280~340nm. The added amount usually does not exceed 5%.
