2,4,6-Trihydroxybenzaldehyde
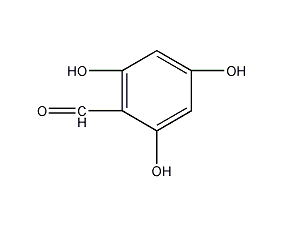

Structural formula
| Business number | 053E |
|---|---|
| Molecular formula | C7H6O4 |
| Molecular weight | 154.12 |
| label |
Phloroglucinolcarboxaldehyde, (HO)3C6H2CHO |
Numbering system
CAS number:487-70-7
MDL number:MFCD00003329
EINECS number:207-663-7
RTECS number:CU8440000
BRN number:2254429
PubChem number:24900385
Physical property data
1. Properties: needle-like crystals
2. Density (g/m3, 25/4℃): Undetermined
3. Relative vapor density (g/cm3, air=1): Undetermined
4. Melting point (ºC, decomposition): 195
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8 . Flash point (ºF): Not determined
9. Specific rotation (º): Not determined
10. Autoignition point or ignition temperature (ºC): Not determined
p>
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ /mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water Log value of (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V /V): Undetermined
19. Solubility: Undetermined
Toxicological data
Acute toxicity: rat oral LD50: 3200mg/kg, tumor-has anti-cancer activity.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 38.65
2. Molar volume (cm3/mol): 96.3
3. Isotonic specific volume (90.2K ): 297.3
4. Surface tension (dyne/cm): 90.5
5. Polarizability (10-24cm3): 15.32
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 15
6. Topological molecule polar surface area 77.8
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 135
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain.�Number of stereocenters of atoms: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Acicular crystals. The color becomes darker after heating, and turns red when exposed to ferric chloride.
It is irritating.
Storage method
Stored sealed and protected from light
Synthesis method
Purpose
Organic Synthesis.
