2,6-Dimethyl-4-heptanol 2,6-Dimethyl-4-heptanol
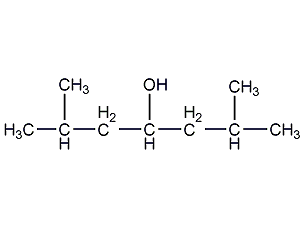

Structural formula
| Business number | 02XZ |
|---|---|
| Molecular formula | C9H20O |
| Molecular weight | 144.25 |
| label |
2,6-dimethyl-4-heptanol, diisobutylmethanol, 2,6-Methyl-4-heptanol, Diisobutyl carbinol, isonyl alcohol, 4-Hydroxy-2,6-dimethylheptane, 2,6-Dimethyl-4-heptanol, 2,6-Dimethyl-heptan-4-ol, Edible spices, alcohol solvent |
Numbering system
CAS number:108-82-7
MDL number:MFCD00008944
EINECS number:203-619-6
RTECS number:MJ3325000
BRN number:None
PubChem number:24901485
Physical property data
1. Properties: Colorless liquid, slightly fragrant.
2. Relative density (g/mL, 20/20℃): 0.8121
3. Relative density (20℃, 4℃): 0.8097
4. Melting point (ºC): -65
5. Boiling point (ºC, normal pressure): 178.0
6. Refractive index (n20ºC): 1.4231
7. Flash point (ºC, opening): 80
8. Viscosity (mPa·s, 0ºC): 56.0
9. Viscosity (mPa·s, 20ºC): 14.3
10. Flash point (ºC): 165
11. Specific heat capacity (KJ/(kg·K)): 2.36
12. Volume expansion coefficient (K-1): 0.00091
13. Heat of evaporation (KJ/mol, 37.8ºC): 51.29
14. Critical temperature (ºC): Undetermined
15. Liquid phase standard hot melt (J·mol-1·K-1): 330.0
16. Solubility (%, 20ºC, water): 0.06
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined Confirm
19. Solubility: Almost insoluble in water, soluble in ethanol and ether.
20. Relative density (25℃, 4℃): 0.8061
21. Refractive index at room temperature (n25): 1.4211
Toxicological data
1. Acute toxicity: rat oral LD50: 3560mg/kg; rat abdominal LD50: 800mg/kg; mouse oral LD50: 3530mg/kg; rabbit skin contact LD50: 4600mg/kg;
p>
Ecological data
None
Molecular structure data
1. Molar refractive index: 45.15
2. Molar volume (cm3/mol): 175.7
3. Isotonic specific volume (90.2K ): 399.0
4. Surface tension (dyne/cm): 26.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 17.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 66.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Flammable liquids. It is non-corrosive to metals and has the chemical reactivity of secondary alcohols.
Storage method
Can be stored in iron, mild steel, copper or aluminum containers.
Synthesis method
It is produced by the catalytic hydrogenation of diisobutyl ketone.
Purpose
GB 2760–1997 stipulates food spices that are allowed to be used. It is used as a solvent for resins such as urea and melamine, and is also used in the manufacture of defoaming agents, lubricant additives, plasticizers, etc.
