3-cyclobutene sulfone 3-Sulfolene
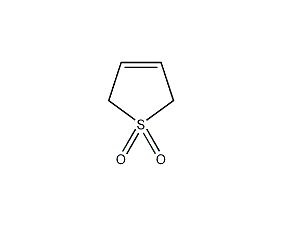

Structural formula
| Business number | 01M7 |
|---|---|
| Molecular formula | C4H6O2S |
| Molecular weight | 118.15 |
| label |
3-Thienene dioxide, 3-Sulfonated cyclopentene, butadiene sulfone, sulfonene, Cyclobutenyl sulfone, 2,5-Dihydrothiophene 1,1-dioxide, 1-thia-3-cyclopentene 1,1-dioxide, 2,5-Dihydrothiophene sulfone, beta-Sulfolene, 1-dioxide, Butadiene sulfone |
Numbering system
CAS number:77-79-2
MDL number:MFCD00005481
EINECS number:201-059-7
RTECS number:XM9100000
BRN number:107004
PubChem number:24888550
Physical property data
1. Character: colorless crystal
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, Air=1): Uncertain
4. Melting point (ºC): 64-65.5℃
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): 1129. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa , 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15 . Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V ): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: soluble in water and organic solvents
Toxicological data
Acute toxicity:
Oral LD50 1600mg/kg(mus)
2547mg/kg(rat)
Skin LD50 >5000mg/kg(rbt)
Main irritating effects:
On the skin: Irritation to skin and mucous membranes.
On eyes: Strong irritation and risk of serious eye injury.
The impact of stimulation.
CongratulationsEffects: No known sensitizing effects.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is slightly hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 27.24
2. Molar volume (cm3/mol): 88.5
3. Isotonic specific volume (90.2K ): 224.1
4. Surface tension (dyne/cm): 41.0
5. Polarizability (10-24cm3): 10.80
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.2
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 42.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 156
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product is sealed and stored in a cool, dry place.
Synthesis method
1. Butadiene, sulfur dioxide and an appropriate amount of hydroquinone are reacted in a reactor at 100°C for 12 hours. The crude product is recrystallized with hot water at 50°C to obtain cyclobutenyl sulfone. Yield 80-85%.
2. Butadiene and sulfur dioxide are in a molar ratio of 1:2 and 1% hydroquinone (calculated as butadiene) to prevent the formation of Polymerization of cycloalkenesulfone) in a steel reactor at 100°C for 12 hours, or in a pressure bottle at room temperature for 2 to 3 weeks. The crude product was recrystallized three times with hot water at 50°C. First time using activated carbon to decolorize. Yield 80-85%. Melting point 64.5-65℃.
If this reaction is carried out in the presence of emulsifier at 20 kg/cm2 (gauge pressure) and 100°C, the reaction can be completed in 1.5 hours, with a yield of 98%
If the purity of commercially available products If it is not enough, it can be treated with activated carbon in its methanol solution (250 ml of methanol and 2 g of activated carbon for every 100 g of crude product). Filter to obtain colorless and odorless crystals
Purpose
1. Used in organic synthesis. 2. The ion source of cis-butadiene is used for DielsAlder reaction. Also known as “diene synthesis” 3.Butadiene source. When heated to 110-130°C, SO2 is lost and pure butadiene is obtained.
