3-Hydroxy-N-(2-methoxyphenyl)-2-naphthylcarboxamide
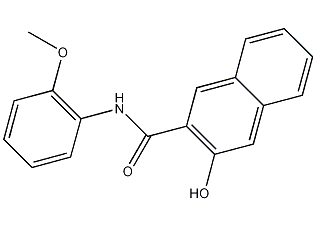

Structural formula
| Business number | 03QB |
|---|---|
| Molecular formula | C18H15NO3 |
| Molecular weight | 293.32 |
| label |
Naphto AS-OL, Naphthol AS-OL, Naphthol AS-OL, Naphthol AS-OL, naphthol, Naphthol AS-OL, 2-(3-Hydroxy-2-naphthamido)anisole, 2′-Methoxy-2-hydroxy-3-naphthanilide, aromatic compounds |
Numbering system
CAS number:135-62-6
MDL number:MFCD00021630
EINECS number:205-206-6
RTECS number:None
BRN number:None
PubChem ID:None
Physical property data
None
Toxicological data
None
Ecological data
None
Molecular structure data
Molecular property data: 1、 Molar refractive index87.23 2、 Molar volume(m3/mol): 224.8 3、 Isotonic specific volume(90.2K):622.4 4、 Surface tension(3.0 dyne/cm):58.7 5、 Polarizability0.5 10-24 cm3):34.58
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 9
6. Topological molecule polar surface area 58.6
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 387
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product is beige brown powder with a melting point of 167-168°C. Insoluble in water, slightly soluble in sodium carbonate solution, soluble in ethanol, and yellow in sodium hydroxide solution.
Storage method
None
Synthesis method
The finished product is obtained by forming a salt from 2,3-acid and chlorobenzene; sodium carbonate; dehydration, condensation with anthranilate in the presence of phosphorus trioxide, neutralization with sodium carbonate, evaporation of chlorobenzene, and then filtration; drying. . Raw material consumption (kg/t) 2,3-acid 710 anthranilate 470 phosphorus trichloride 270 sodium carbonate (98%) 570 chlorobenzene (98%) 150 insurance powder 8
Purpose
It is mainly used as a primer for cotton fabric dyeing and printing. It can also be used to make fast pigments and as an intermediate for organic pigments.
tyle=”FONT-SIZE: 9pt; FONT-FAMILY: Arial; mso-fareast-font-family: Arial; mso-font-kerning: 0pt”>10-24cm3): 34.58
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 9
6. Topological molecule polar surface area 58.6
7. Number of heavy atoms: 22
8. Surface charge: 0
9. Complexity: 387
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product is beige brown powder with a melting point of 167-168°C. Insoluble in water, slightly soluble in sodium carbonate solution, soluble in ethanol, and yellow in sodium hydroxide solution.
Storage method
None
Synthesis method
The finished product is obtained by forming a salt from 2,3-acid and chlorobenzene; sodium carbonate; dehydration, condensation with anthranilate in the presence of phosphorus trioxide, neutralization with sodium carbonate, evaporation of chlorobenzene, and then filtration; drying. . Raw material consumption (kg/t) 2,3-acid 710 anthranilate 470 phosphorus trichloride 270 sodium carbonate (98%) 570 chlorobenzene (98%) 150 insurance powder 8
Purpose
It is mainly used as a primer for cotton fabric dyeing and printing. It can also be used to make fast pigments and as an intermediate for organic pigments.
