4-Acetamidobenzenesulfonyl chloride 4-Acetamidobenzenesulfonyl chloride
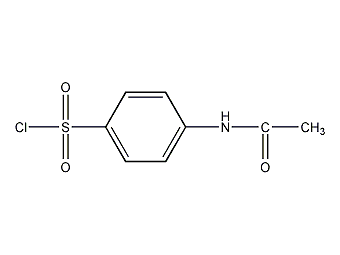
Structural formula
| Business number | 03DT |
|---|---|
| Molecular formula | C8H8ClNO3S |
| Molecular weight | 233.67 |
| label |
p-acetamidobenzenesulfonyl chloride, 4-acetamidobenzenesulfonyl chloride, N-acetyl sulfonamide chloride, Acetaminophenyl chloride, N-acetyl p-aminobenzenesulfonyl chloride, -N-acetyl p-aminobenzenesulfonyl chloride, N-acetyl sulfonamide chloride, 4-acetamidobenzenesulfonyl chloride, P-Acetaminobenzenesulfonyl chloride, P-Acetamidobenzenesulfonylchloride, NASC, N-Acetylsulfanilic acid Chloride, N-Acetylsulfanilyl chloride, aromatic sulfur compounds |
Numbering system
CAS number:121-60-8
MDL number:MFCD00007442
EINECS number:204-485-1
RTECS number:DB8837500
BRN number:746676
PubChem ID:None
Physical property data
1. Properties: light brown to brown powder or fine crystals
2. Melting point (decomposition, ℃): 145~148
3. Solubility: easily soluble in ethanol , diethyl ether, soluble in hot benzene and hot chloroform, decomposed in water. Needle-shaped crystals are precipitated from benzene, and prismatic crystals are precipitated from benzene-chloroform.
Toxicological data
1. Acute toxicity: Rat oral LD50: >3200mg/kg
Rat peritoneal cavity LD50: 25mg/kg
Mouse peritoneal cavity LDL0: 50mg /kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 53.42
2. Molar volume (cm3/mol): 159.1
3. Isotonic specific volume (90.2K): 433.9
4. Surface tension (dyne/cm): 55.2
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 21.17
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 71.6
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 301
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain chemical bond establishment�Number of centers: 0
15. Number of covalent bond units: 1
Properties and stability
1. It easily absorbs moisture and decomposes in the air. Has a slight acetic acid smell.
2.It is corrosive and toxic. Irritating to skin and mucous membranes. During the production process, attention should be paid to protective measures to prevent accidental ingestion. Operators should wear protective equipment. Do not stick to skin.
Storage method
1. This product is an organic intermediate. It is easy to absorb moisture and cause decomposition, so it is not suitable for long-term storage. It is generally used to prepare other products immediately after being produced by the manufacturer.
2. Store in a nitrogen-filled seal.
Synthesis method
Chlorosulfonation of Acetanilide Below are two examples of industrial operations. Operation Example 1 Add 67.5kg acetamidobenzene to 290kg chlorosulfonic acid under stirring, control the adding temperature below 15°C, gradually raise the temperature to 60°C after addition, keep stirring for 2 hours, and then gradually add the reactant to ice water to dilute , keep the temperature below 10°C, filter, and rinse with ice water until the Congo red test paper becomes neutral. Obtain acetaminophenyl chloride. Operation Example 2 Add acetamidobenzene evenly to chlorosulfonic acid below 20°C. By weight, acetanilide: chlorosulfonic acid = 1:5.26, react at 50±2°C for 3 hours. After standing for 8 hours, at about 20°C or below, add an appropriate amount of water to decompose the excess chlorosulfonic acid. The temperature does not exceed 28°C. Cool slightly, filter, and wash the filter cake to pH=3-4 to obtain acetamidosulfonyl chloride. The yield is 83%. This product should not be stored for a long time. As an intermediate product in the production of medicine and dyes, the content is 30%.
![]()
Purpose
Intermediates for a variety of sulfa drugs, such as sulfathiazole, sulfisoxazole, sulfamethoxazole, sulfaphenazole, sulfamethoxazole, etc. It is also an intermediate of dyes.
