4-Aminophenol hydrochloride 4-Aminophenol hydrochloride
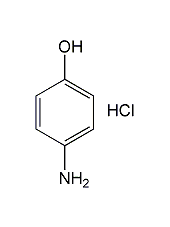

Structural formula
| Business number | 0156 |
|---|---|
| Molecular formula | C6H8ClNO |
| Molecular weight | 145.59 |
| label |
Para-aminophenol hydrochloride, paraaminophenol hydrochloride, 4-aminophenol hydrochloride, Paraaminophenol hydrochloride, 4-Hydroxyaniline hydrochloride, p-Aminophenol hydrochloride, p-Hydroxyaniline hydrochloride |
Numbering system
CAS number:51-78-5
MDL number:MFCD00012996
EINECS number:200-122-6
RTECS number:SJ6070000
BRN number:3911747
PubChem number:24891061
Physical property data
1. Properties: White crystalline powder. The light gradually turns black.
2. Density (g/mL, 25/4℃): 1.5113. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 306 (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined Determined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition Temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water and ethanol, slightly soluble in ether.
Toxicological data
1. Acute toxicity: Mouse abdominal cavity LD50: 750mg/kg 2. Mutagenicity: Specific trajectory test: Insect-Drosophila Oral: 20mmol/L
Ecological data
None
Molecular structure data
1. Molar refractive index: 32.37
2. Molar volume (cm3/mol): 90.1
3. Isotonic specific volume (90.2K ): 248.1
4. Surface tension (dyne/cm): 57.4
5. Polarizability (10-24cm3): 12.83
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP):���
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 4
6. Topological molecule polar surface area 46.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 66.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters :0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds Number of structural centers: 0
15. Number of covalent bond units: 2
Properties and stability
1. Harmful if inhaled, taken orally or in contact with skin.
Storage method
1. Store sealed and protected from light.
Synthesis method
The product is prepared by reducing p-nitrophenol with iron powder (or sodium sulfide), filtering, acid precipitation, dehydration and drying. The product is required to be brown to tan powder, the color is not similar to the standard product, the strength is 100±3 (minutes) of the standard product, and the content is 70±2%. Dyeing fastness: no sun exposure and friction when dyeing with mordant or iron coal. The fastness is all level 4; when copper mordanting, the sun exposure level is 5-6, and the rubbing level is 4; when chromium mordanting, the sun exposure level is 6, and the rubbing level is 4-5.
Purpose
1. Organic synthesis. Take photos. Mainly used for dyeing fur brown, and can also be used as sulfur dyes and pharmaceutical intermediates.
