4-Butylaniline 4-Butylaniline
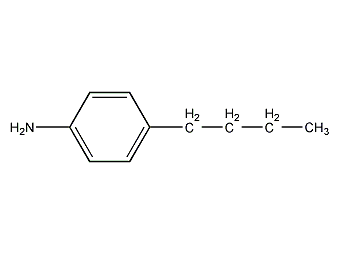

Structural formula
| Business number | 02PR |
|---|---|
| Molecular formula | C10H15N |
| Molecular weight | 149.23 |
| label |
p-n-butylaniline, 4-n-Butylaniline, 4-Butylaniline, p-butylaniline, 4-N-Butylaniline, p-Butylaniline, p-N-Butylaniline, 4-Amino-1-butylbenzene, 4-Butylaniline, 4-N-Butylaniline, Nitrogen-containing compound solvents, aromatic compounds |
Numbering system
CAS number:104-13-2
MDL number:MFCD00007925
EINECS number:203-177-4
RTECS number:BW9470000
BRN number:1447268
PubChem number:24847105
Physical property data
1. Properties: colorless or light yellow liquid with ammonia smell.
2. Density (g/mL, 20/4℃): 0.93226
3. Density (g/mL, 25℃): 0.92835
4 . Density (g/mL, 30℃): 0.92444
5. Melting point (ºC): -14.40
6. Boiling point (ºC, normal pressure): 241.59
7. Boiling point (ºC, 1.86kpa): 133-134
8. Refractive index (20ºC): 1.53412
9. Refractive index (25ºC): 1.53167
p>
10. Refractive index (30ºC): 1.52935
11. Viscosity (mPa·s, 20ºC): 3.2237
12. Flash point (ºC, opening): 107
13. Vapor pressure (kPa, 69.59ºC): 0.13
14. Vapor pressure (kPa, 111.65 ºC): 1.33
15. Vapor pressure (kPa, 136.69ºC): 4.00
16. Vapor pressure (kPa, 169.26ºC): 13.33
17. Solubility: insoluble in water, soluble in ethanol, ether and acetone , carbon tetrachloride, benzene, heptane, etc. are miscible.
Toxicological data
1. Acute toxicity: mouse peritoneal cavity LD50: 81mg/kg; 2. Other multiple dose toxicity: rabbit skin contact TDLo: 3 mL/kg/3D-I;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 49.30
2. Molar volume (cm3/mol): 157.5
3. Isotonic specific volume (90.2K ): 389.2
4. Surface tension (dyne/cm): 37.2
5. Dielectric constant:
6. Dipole moment���10-24cm3):
7. Polarizability: 19.54
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 93
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides. Paraffin and stearic acid can be dissolved when heated.
Chemical properties: Alkaline and approximately equal to aniline. N-butylaniline salt is easier to dissolve than aniline salt, but it is difficult to crystallize. Easily generate N-acylate. Reacts with nitrous acid to obtain nitrosamines.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire, heat and water sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Derived from the reaction of aniline and butanol in the presence of zinc chloride. Heat 1 mol of aniline, 1 mol of butanol, and 6.5 mol of anhydrous zinc chloride to 121°C with a pressure of 0.78MPa. After 6 hours of reaction, the temperature is raised to 240°C with a pressure of 2.06-2.16MPa. The reaction lasts for 10 hours to the end point. The reactant was refluxed in alkali solution for 5 hours, and 4.5-5kg of crude product was separated, with a yield of 40-45%.
Purpose
Dye intermediates. Used to produce Kaplan Blue, Green GS, 5GS, etc.
