4-Heptanone 4-Heptanone
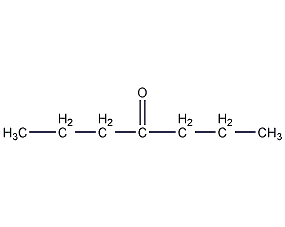
Structural formula
| Business number | 03GA |
|---|---|
| Molecular formula | C7H14O |
| Molecular weight | 114.19 |
| label |
Methyl amyl ketone, lactoketone, dipropyl ketone, heptanone, Propyl ketone, Dipropyl ketone, Butyrone, food additives, Flavor enhancer |
Numbering system
CAS number:123-19-3
MDL number:MFCD00009403
EINECS number:204-608-9
RTECS number:MJ5600000
BRN number:1699049
PubChem number:24869929
Physical property data
1. Properties: Colorless and transparent liquid with strong ethereal and fruity aroma, including cheese, jackfruit, etc.
2. Boiling point (ºC, 101.3kPa): 144.0
3. Melting point (ºC): -33
4. Relative density (g/mL, 20/4ºC): 0.8145
5. Refractive index (21.7ºC): 1.40732
6. Viscosity (mPa·s, 25ºC): 0.685
7 . Flash point (ºC, ASTM, open): 48
8. Heat of evaporation (KJ/kg, b.p.): 317
9. Heat of formation (KJ/mol, gas) :298.52
10. Heat of combustion (KJ/mol): 4400
11. Specific heat capacity (KJ/(kg·K), 20~40ºC, constant pressure): 2.310
p>
12. Body expansion coefficient (K-1, about 20ºC): 0.001073
13. Body expansion coefficient (K-1 , about 55ºC): 0.001115
14. Solubility: Miscible with various organic solvents such as alcohols and ethers. It can dissolve raw rubber, nitrocellulose, grease, natural resin and various vinyl synthetic resins. It dissolves 0.43% in water at 20℃; water dissolves 0.87% in 4-heptanone.
15. Relative density (20℃, 4℃): 0.8160
16. Relative density (25℃, 4℃): 0.8116
17. Normal temperature Refractive index (n20): 1.4067
18. Refractive index at room temperature (n25): 1.4045
19. Critical Temperature (ºC): 328.85
20. Critical density (g·cm-3): 0.263
21. Critical volume (cm3 ·mol-1): 434
22. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -4457.13
23. The liquid phase standard claims heat (enthalpy) (kJ·mol-1): -298.32
Toxicological data
1. Acute toxicity: Rat oral LD50: 3730uL/kg; Rat inhalation LC50: 2690ppm/6H
Mouse intravenous LDLO: 271mg/kg; rabbit skin LD50: 5660uL/kg
2. Other multiple dose toxicity: rat oral TDLO: 30gm/kg/3W-I; rat oral TDLO : 130gm/kg/13W-I
3. The toxicity caused by oral or skin absorption is very small, but it is irritating to mucous membranes.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 34.50
2. Molar volume (cm3/mol): 141.2
3. Isotonic specific volume (90.2K): 315.7
4. Surface tension (dyne/cm): 24.9
5. Polarizability (10-24cm3): 13.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 58.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is easy to cause combustion when exposed to high heat, open flames or strong oxidants.
2. Exist in smoke.
3. Naturally found in apple juice, papaya, pears, baked potatoes, bread, coffee, peanuts, etc.
Storage method
Keep away from open flames or strong oxidants.
Synthesis method
1. Refining method: Often mixed with other ketones, it is generally refined by distillation. You can also add silver ammonium nitrate solution and stir, let it stand for 30 minutes until it no longer develops color, then wash with water, dry with anhydrous calcium sulfate and then distill.
2. Butyric acid is prepared by precipitating calcium carbonate at 450ºC.
3. Preparation method:
![]()
Refer to the above preparation method of 3-pentanone (reference book 335), react with 880g (10mol) of n-butyric acid (2) to obtain 285g of 4-heptanone (1), bp 142~143℃, collected The rate is 46%. [1]
4. Preparation method:
![]()
Contains formaldehyde. In the reaction bottle of the reflux condenser, add 123g (2.2mol) of reduced iron powder and 370mL of n-butyric acid (2), stir and heat to reflux for 5 hours. Change to a distillation device, heat and distill under nitrogen protection, and stop supplying nitrogen at a later stage. Intense heat distills out everything that can be distilled out. The crude product was washed with 10% sodium hydroxide solution and water in sequence, dried over anhydrous sodium sulfate, and after rotation and concentration, the residue was rectified and the fractions at 142-144°C were collected to obtain 157-171g of 4-heptanone (1). The rate is 69%~75%. [2]
Purpose
It is mainly used as a solvent for nitrocellulose, nitrocellulose paint and synthetic resin, as well as a raw material for organic synthesis.
