Isopentyl Formate Isopentyl Formate
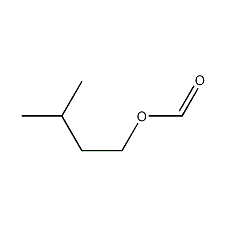

Structural formula
| Business number | 0318 |
|---|---|
| Molecular formula | C6H12O2 |
| Molecular weight | 116.16 |
| label |
Isoamyl formate, 3-Methyl-1-butanol formate, 3-Methylbutyl formate, food additives, Flavor enhancer |
Numbering system
CAS number:110-45-2
MDL number:MFCD00021049
EINECS number:203-769-2
RTECS number:NT0185000
BRN number:1739893
PubChem number:24900836
Physical property data
1. Properties: colorless to yellow flammable liquid with fruity aroma. [1]
2. Melting point (℃): -93.5[2]
3. Boiling point (℃): 123~124[3]
4. Relative density (water=1): 0.877[4]
5. Relative vapor density (air = 1): 4[5]
6. Saturated vapor pressure (kPa): 1.33 (17℃)[6]
7. Octanol/water partition coefficient: 1.72[7]
8. Flash point (℃): 30.0 (CC) [8]
9. Explosion upper limit (%): 8.0[9]
10. Explosion lower limit (%): 1.2[10]
11. Solubility: Slightly soluble in water, soluble in most organic solvents. [11]
12. Refractive index (n20ºC): 1.391
13. Refractive index (n25ºC): 1.3940
Toxicological data
1. Irritation: Rabbit skin standard Drez eye dye test: 500mg/24H Moderate irritation.
2. Acute toxicity: rat oral LD50: 9840mg/kg; rabbit oral LD50: 3020mg/kg; rabbit dermal LD50: >5gm/kg
3. Vapor It has an irritating effect on the mucous membranes of the eyes, nose and throat, and high-concentration vapor has an anesthetic effect.
4. Acute toxicity[12] LD50: 9840mg/kg (rat oral); >5g/ kg (rabbit transdermal)
5. Irritation[13] Rabbit transdermal 500mg (24h), Moderately irritating.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[14] This substance May be harmful to the environment, special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 31.73
2. Molar volume (cm3/mol): 131.6
3. Isotonic specific volume (90.2K ): 296.7
4. Surface tension (dyne/cm): 25.8
5. Polarizability (10-24cm3): 12.58
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: None
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 59.5
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[15] Stable
2. Incompatible substances [16] Strong oxidants, strong acids, strong bases, halogens
3. Polymerization hazards[17] No aggregation
Storage method
Storage Precautions[18] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, and halogens, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Add isoamyl alcohol and sulfuric acid to formic acid and esterify it.
2. Obtained from esterification of isoamyl alcohol (or fusel oil) and sodium formate in the presence of concentrated sulfuric acid and then fractionation.
3. The finished product isoamyl formate is obtained by reacting 1245kg of formic acid (95%) and 500kg of isoamyl alcohol (95%) in the presence of sulfuric acid, followed by neutralization with alkali, dehydration and rectification.
Purpose
1. Used as a solvent for nitrocellulose and paint.
2. Used for making sherbet and other spices, as well as organic synthesis and solvents.
3. Used as an organic solvent for the manufacture of spices, fumigation pesticides and fungicides. [19]
