n-Propyl Formate n-Propyl Formate
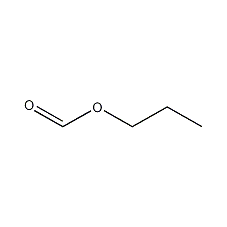

Structural formula
| Business number | 031Z |
|---|---|
| Molecular formula | C4H8O2 |
| Molecular weight | 88.11 |
| label |
n-propyl formate, propyl formate, 1-Propylformate, n-Propyl methanoate, paint solvents, dispersants for pesticides, For immune specificity research |
Numbering system
CAS number:110-74-7
MDL number:MFCD00003295
EINECS number:203-798-0
RTECS number:LR0175000
BRN number:1739272
PubChem number:24901411
Physical property data
1. Properties: colorless liquid with special fragrance. [1]
2. Melting point (℃): -92.9[2]
3. Boiling point (℃): 81.3[3]
4. Relative density (water = 1): 0.901[4]
5. Relative vapor Density (air=1): 3.03[5]
6. Saturated vapor pressure (kPa): 6.65 (20℃)[6]
7. Critical pressure (MPa): 4.06[7]
8. Octanol/water partition coefficient: 0.83[8]
9. Flash point (℃): -2.8 (CC) [9]
10. Ignition temperature (℃): 455[10]
11. Explosion upper limit (%): 11.3[11]
12. Explosion lower limit (%): 2.1[12]
13. Solubility: Slightly soluble in water, soluble in most organic solvents. [13]
14. Viscosity (mPa·s, 15ºC): 0.544
15. Heat of evaporation (KJ/mol, 80.85ºC): 32.51
16. Specific heat capacity (KJ/(kg·K), 9~57ºC, constant pressure): 2.03
17. Electrical conductivity (S/m, 17ºC): 5.5×10 -5
18. Thermal conductivity (W/(m·K), 12ºC): 0.1495
19. Relative density (20℃, 4 ℃): 0.9055
20. Relative density (25℃, 4℃): 0.8996
21. Refractive index at room temperature (n25): 1.3750
22. Critical density (g·cm-3): 0.309
23. Critical volume (cm3·mol-1): 285
24. Critical compression factor: 0.259
25. Eccentricity factor: 0.318
26. Solubility parameter (J·cm-3)0.5: 18.340
27. van der Waals area (cm2·mol-1): 7.790×109
28. van der Waals volume (cm3·mol– 1): 52.970
29. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2254.6
30. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -448
31. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1 ): -2217.1
32. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -484
33 .Liquid phase standard hot melt (J·mol-1·K-1): 171.9
Toxicological data
1. Acute toxicity: Mouse oral LD50: 3400mg/kg; Rat oral LD50: 3980mg/kg; Rabbit skin LD50: 360ul/kg; Mammalian oral LD50: 1200mg/kg;
2. The toxicity is slightly lower than that of ethyl acetate, and its vapor is harmful to the eyes., nose and tongue have a strong stimulating effect. The maximum allowable concentration in the workplace is 360mg/m3 (0.36mg/L air).
3.Acute toxicity[14]
LD50: 3980mg/kg (rat Oral); 3400mg/kg (oral for mice)
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
4. Other harmful effects[15] This substance May be harmful to the environment, special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 22.51
2. Molar volume (cm3/mol): 98.2
3. Isotonic specific volume (90.2K ): 219.8
4. Surface tension (dyne/cm): 25.0
5. Polarizability (10-24cm3): 8.92
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 34.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[16] Stable
2. Incompatible substances [17] Strong oxidants, strong bases, strong acids, halogens
3. Polymerization hazards[18] No aggregation
Storage method
Storage Precautions[19] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, and halogens, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Refining method: often contains free formic acid and propanol. During refining, it is washed with sodium carbonate aqueous solution, dried with potassium carbonate or phosphorus pentoxide and then fractionated. It can also be distilled once first, and the distillate is washed with a saturated aqueous sodium chloride solution, then washed with a saturated aqueous sodium bicarbonate solution containing solid sodium chloride, dried with anhydrous magnesium sulfate and then fractionated.
Purpose
1. Research on immune specificity. Substrate for colorimetric quantitative analysis of proteolytic enzymes. Used as paint solvent, pesticide dispersant, organic synthesis raw material, etc. Also used in the manufacture of spices, artificial leather, safety glass, etc.
2. Used as organic solvents and used in the manufacture of spices, fumigation pesticides and fungicides. [20]
