2-Octanone 2-Octanone
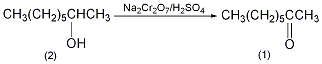
Structural formula
| Business number | 032S |
|---|---|
| Molecular formula | C8H16O |
| Molecular weight | 128.21 |
| label |
Secondary Octanone, Methylhexyl Ketone, n-hexyl methyl ketone, Methylhexanone, n-Hexyl methyl ketone, Hexyl methyl ketone, Methyl hecxyl ketone, defoaming agent, Surfactant, flotation agent, food additives, Flavor enhancer |
Numbering system
CAS number:111-13-7
MDL number:MFCD00009540
EINECS number:203-837-1
RTECS number:RH1484000
BRN number:635843
PubChem number:24845301
Physical property data
1. Properties: colorless to light yellow liquid with floral and grassy aroma, accompanied by the fragrance of mignonette.
2. Density (g/mL, 20℃): 0.8192
3. Density (g/mL, 25℃): 0.8151
4. Relative Vapor density (g/mL, air=1): 4.4
5. Melting point (ºC): -16
6. Boiling point (ºC, normal pressure): 173
7. Refractive index(n20D): 1.415
8. Refractive index (n25ºC) : 1.4133
9. Flash point (ºC, closed cup): 62
10. Viscosity (mPa·s, 20ºC): 1.02
11. Liquid Phase standard hot melt (J·mol-1·K-1): 274.7
12. Vapor pressure (kPa, 20 ºC): < 0.13
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): 359.55
15. Critical pressure (KPa) : Undetermined
16. Relative evaporation rate (ether=1): 65
17. Critical density (g·cm-3): 0.258
18. Critical volume (cm3·mol-1): 497
19. Solubility: slightly soluble in water , the solubility in water at 20°C is 0.09%, and the solubility of water in 2-octanone is 0.6%. Miscible with organic solvents such as ether and alcohol.
20. Relative density (20℃, 4℃): 0.8202
21. Solubility parameter (J·cm-3)0.5 : 19.152
22. van der Waals area (cm2·mol-1): 1.259×1010
23. van der Waals volume (cm3·mol-1): 90.190
Toxicological data
1. Acute toxicity: rat�LD50: 3089mg/kg; rat inhalation LC50: >2132ppm/6H
mice Abdominal LD50: 800mg/kg; Rabbit skin LD50: 1337mg/kg
2. Irritation: Rabbit transdermal standard Drez eye dye test: 500mg/24H Mild irritation.
3. Reproductive toxicity: intraperitoneal TDLO in female mice after fertilization: 1gm/kg for 1-20 days
Ecological data
This substance is harmful to the environment, and special attention should be paid to atmospheric pollution.
Molecular structure data
1. Molar refractive index: 39.14
2. Molar volume (cm3/mol): 157.7
3. Isotonic specific volume (90.2K ): 355.4
4. Surface tension (dyne/cm): 25.8
5. Polarizability (10-24cm3): 15.51
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 3
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 76.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It does not decompose under normal temperature and pressure. It is forbidden to come into contact with strong oxidants, strong reducing agents and strong alkali. Flammable liquids should be treated as flammable products.
2. Found in tobacco leaves.
3. Small amounts naturally occur in rue oil and banana and citrus fruits.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. It is formed from the oxidation of sec-octanol, the cracked product of castor oil.
2. Preparation method:
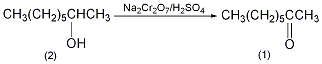
Into a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and dropping funnel, add 65g (0.5mol) of 2-octanol (2) and 200mL of diethyl ether, and cool in an ice-water bath. Add 250 mL of chromic acid solution ① dropwise, keeping the reaction temperature at 25~30°C, and complete the addition in about 20 minutes. The reaction was then continued to stir at room temperature for 2 h. Separate the ether layer, extract the water layer with ether (80 mL × 4), combine the ether layers, wash with saturated sodium bicarbonate and saturated sodium chloride solution in sequence, and dry over anhydrous sodium sulfate. Steam off the ether. Then, it was distilled under normal pressure, and the fractions at 170-172°C were collected to obtain 53g of 2-octanone (1) with a yield of 81%. The chromic acid solution can be prepared as follows: 100g (0.33mol) sodium dichromate dihydrate is dissolved in 300mL of water, slowly add 73mL of sulfuric acid (98%), and dilute to 500mL with water after cooling. [1]
3. Preparation method:
In a reaction bottle equipped with a stirrer and a reflux condenser, Add 100 mL of formic acid and 7.5 g of octyne-1(2), heat in an oil bath, and stir the reaction at 100°C until the reaction of the starting materials is basically complete. The reaction was followed by gas chromatography during the reaction. When the reaction lasted for about 6 hours, quantitative gas chromatography analysis showed that 92% of 2-octanone was produced. Cool, extract with dichloromethane, wash with water, sodium carbonate solution, water in sequence, and dry over anhydrous magnesium sulfate. Filter, concentrate under reduced pressure and then distill. Collect the fraction at 171-173°C to obtain 7.42g of compound (1) with a yield of 85%. [2]
Purpose
1. Used in the fields of fiber, medicine, pesticides, fragrance chemicals, etc., as synthetic fiber oil agent, defoaming agent and preparation of surfactant, flotation agent for coal mines, etc. It is used as a solvent for vinyl compounds and dyes, to extract phenols from water, and to separate gallium from aluminum. It is especially used in printing inks with disperse dyes.
2. Can be used for artificial essential oils.
