m-Aminobenzenesulfonic acid m-Aminobenzenesulfonic acid
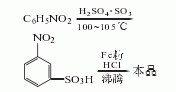
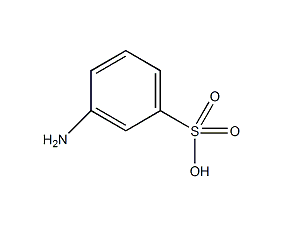
Structural formula
| Business number | 03DN |
|---|---|
| Molecular formula | C6H7NO3S |
| Molecular weight | 173.19 |
| label |
m-aminobenzenesulfonic acid, Aniline sulfonic acid, meta-amino acid, 3-aminobenzenesulfonic acid, interacid, meta-aminobenzene sulfonic acid, m-sulfamic acid, 3-Aminobenzenesulfonic acid, M-sulfanilic acid, M-sulfoaniline, 1-Aminobenzene-3-sulfonic acid, Metanilic acid, aromatic sulfur compounds |
Numbering system
CAS number:121-47-1
MDL number:MFCD00065345
EINECS number:204-473-6
RTECS number:OY2300000
BRN number:473264
PubChem number:24845997
Physical property data
1. Properties: white flaky crystals, industrial products are gray with pink paste.
2. Relative density: 1.69
3. Melting point (℃): 288
4. Solubility: slightly soluble in ethanol, ether, soluble in water , lye and concentrated hydrochloric acid.
Toxicological data
1. Mutagenicity: microbial mutation testing system: 1pph
Ecological data
None
Molecular structure data
1. Molar refractive index: 40.71
2. Molar volume (cm3/mol): 114.4
3. Isotonic specific volume (90.2K): 328.2
4. Surface tension (dyne/cm): 67.5
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 16.14
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.2
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 80.4
p>
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 219
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
This product is highly toxic and its toxicity is similar to that of aniline.
Decomposes when heated.
Storage method
Packaged in iron drums lined with plastic bags. Each barrel weighs 50kg. Store in a cool, dry and ventilated place, avoid sunlight. Handle with care when handling to prevent heat and moisture. Store and transport according to regulations on highly toxic chemicals.
Synthesis method
It is produced by sulfonation, reduction and acid precipitation of nitrobenzene. The process is as follows:
(1) Sulfonation: 1600L of 20% fuming sulfuric acid is added into the acid steamer at a rate of 500L, h, and the temperature is maintained at about 250°C. The steamed sulfur trioxide vapor is condensed to obtain 285L of liquid sulfur trioxide. The sulfur trioxide was added to the sulfonation pot containing 667kg of nitrobenzene within 3 hours, and the temperature was raised from room temperature to 90°C. After the addition, the temperature was raised to 115°C, and the temperature was maintained for 3 hours before completion. Dilute it in 3000L water or iron mud washing liquid, and neutralize it to neutrality with liquid alkali. Filter to obtain m-nitrobenzene sulfonate sodium salt solution. The yield is 95%.
(2) Reduction: Prepare electrolyte (activation) in a reduction pot by adding one-fifth of the sulfonation diluent (not neutralized with liquid alkali) and 700kg of iron filings. Under direct steam boiling, within about 2-3 hours, add the remaining four-fifths of the neutralizing liquid into the reduction pot. After adding, continue boiling and reduction for 0.5h. After the sampling conversion rate is ≤98%, add liquid alkali to adjust the pH value to 9, filter while hot, and filter the iron sludge. The filtrate is m-aminobenzenesulfonic acid sodium salt solution (after the sodium salt solution is evaporated and concentrated, it can be used as a dye intermediate Body use). The yield is 88%.
(3) Acid precipitation Add sulfuric acid to the sodium salt solution in the acid precipitation pot until the Congo red test paper turns blue, and keep the temperature at 70°C. Then centrifuge and filter to obtain m-aminobenzene sulfonic acid slurry. The yield is 85%. The total yield is 71% (based on nitrobenzene). Specifications of industrial products as dye intermediates: Appearance is off-white to light red crystal or slurry, total amino content is ≥60%, equivalent to dry product content is not less than 90.9%, insoluble matter in soda ash is ≤1.5%.
Raw material consumption quota: nitrobenzene 1035kg/t; liquid alkali (30%) 2106kg/t; oleum 4264kg/t; iron filings 934kg/t.
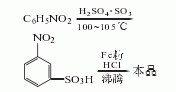
Purpose
1. Used in the manufacture of azo dyes, acid dyes, reactive dyes, sulfur dyes, such as weak acid dark blue 5R, GR acidic golden G, reactive bright orange K-G, K-GN, KR, K-7R, etc.
2. Used in the manufacture of stilbene-based fluorescent whitening agents such as fluorescent whitening agent B, Tianlaibao 2B, Blankophor BBU. It is also used to prepare m-aminophenol, vanillin, etc.
