3′-Hydroxyacetophenone 3′-Hydroxyacetophenone
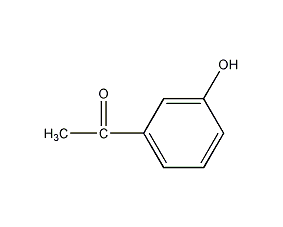

Structural formula
| Business number | 03DY |
|---|---|
| Molecular formula | C8H8O2 |
| Molecular weight | 136.15 |
| label |
m-hydroxyacetophenone, m-Hydroxyacetophenone, m-Acetylphenol, 3-Hydroxyacetophenone, 3-Acetylphenol, m-Acetylphenol, Ethanone, 1-(3-hydroxyphenyl)-, m-Hydroxyacetophenonel, 3-Acetylphenol, m-Acetylphenol, aromatic compounds |
Numbering system
CAS number:121-71-1
MDL number:MFCD00002298
EINECS number:204-494-0
RTECS number:AM8574000
BRN number:2040676
PubChem number:24859714
Physical property data
1. Characteristics: Acicular crystal
2. Relative density (109℃): 1.099
3. Refractive index ( n109D): 1.5348
4. Melting point (℃): 95-97
5. Boiling point (ºC): 296
6. Boiling point (ºC, 0.67kpa): 153
Toxicological data
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 38.16
2. Molar volume (cm3/mol): 119.3
3. Isotonic specific volume (90.2K): 307.4
4. Surface tension (dyne/cm): 43.9
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 6
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 131
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Exist inIn the flowing smoke.
Storage method
None
Synthesis method
2. Brief description of production method
Obtained from the reaction of m-aminoacetophenone and sodium nitrite. Add water and concentrated sulfuric acid into the reaction pot, add m-aminoacetophenone while stirring, and then add sodium nitrite aqueous solution dropwise while cooling, keeping the temperature at 8-10°C. After adding, slowly raise the temperature to above 90°C, and continue stirring. Reaction 1h. The solid is precipitated by cooling, and is filtered. The obtained crude product is recrystallized with hot water to obtain m-hydroxyacetophenone. The yield is 65%.
Purpose
3. Use
It is used in organic synthesis and is an intermediate of the drug phenylephrine.
