Methyl cyanoacetate Methyl cyanoacetate
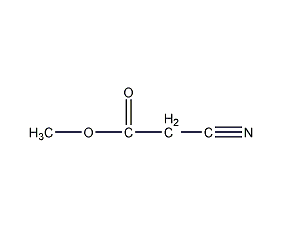
Structural formula
| Business number | 02RH |
|---|---|
| Molecular formula | C4H5NO2 |
| Molecular weight | 99 |
| label |
Methyl cyanoacetate |
Numbering system
CAS number:105-34-0
MDL number:MFCD00001939
EINECS number:203-288-8
RTECS number:AG4375000
BRN number:773945
PubChem ID:None
Physical property data
1. Properties: colorless to slightly yellow transparent liquid, flammable. Slightly aromatic.
2. Density (g/mL, 25/4℃): 1.1225
3. Relative vapor density (g/mL, air=1): 3.41
4. Melting point (ºC): -13.07
5. Boiling point (ºC, normal pressure): 205.09
6. Refractive index (25ºC): 1.41662
7. Viscosity (mPa·s, 20ºC): 2.793
8. Flash point (ºC): 110
9. Autoignition point or ignition temperature ( ºC): 475
10. Heat of evaporation (KJ/mol, 25ºC): 61.713
11. Heat of combustion (KJ/mol, 25ºC): 1992.1
12. Conductivity (S/m): 4.49×10-7
13. Solubility: Slightly soluble in water, miscible with alcohol and ether. Can dissolve a variety of salts.
Toxicological data
1. Acute toxicity: mouse peritoneal cavity LD50: 200mg/kg; guinea pig skin contact LDLo: 400mg/kg; 2. Can be absorbed through the skin and can cause skin inflammation.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 22.26
2. Molar volume (cm3/mol): 91.4
3. Isotonic specific volume (90.2K): 224.3
4. Surface tension (dyne/cm): 36.2
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 8.82
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 50.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 110
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants. Insoluble in water. Miscible with ethanol and ether. Combustible, poisonous. When using, avoid skin contact and inhalation of its vapor.�
Chemical properties: Methyl cyanoacetate contains active methylene and can generate sodium derivatives. It can undergo polycondensation reaction with formaldehyde, acetaldehyde or other aldehydes and ketones. It is less acidic than phenol. It can be hydrolyzed with acid, water and alkali.
2.This product is moderately toxic. The LD50 of guinea pigs administered orally and intraperitoneally is 400 to 800 mg/kg. For protective measures, see Cyanoacetic acid. Operators should wear protective equipment.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. This product is flammable and toxic. It is packaged in sealed aluminum barrels or plastic barrels, 200kg per barrel. Keep away from fire sources during storage and transportation.
Synthesis method
The synthesis of methyl cyanoacetate mainly includes the following types: First, chloroacetic acid reacts with sodium cyanide first, and the generated cyanoacetic acid is then reacted with methyl ester The second one is obtained by esterifying chloroacetic acid with methanol and then reacting with sodium cyanide. The third one is obtained by transesterification of ethyl cyanoacetate and methanol. The following is a brief description of the second synthesis method:
1. Chloroacetic acid and methanol esterification method Perform an esterification reaction between chloroacetic acid and methanol to generate methyl chloroacetate. After rectification, the refined product of methyl chloroacetate is obtained, which is cyanated with sodium cyanide to obtain the crude product. Then, the finished product of methyl cyanoacetate is obtained through filtration, atmospheric distillation, and vacuum distillation.
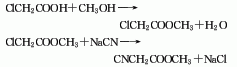
2. Cyanoacetic acid and methyl ester Chemical method: Add cyanoacetic acid, methanol, and sulfuric acid into the reactor, heat to reflux for 3 hours, cool to 10°C, add sodium carbonate solution to neutralize, and distill at 132-136°C and 2.133kPa to obtain the finished product of methyl cyanoacetate.
Refining method: dry with potassium carbonate or anhydrous sodium sulfate and then fractionate.
![]()
3.Synthesis of cyanoethanol methyl ester In a 250mL three-necked bottle equipped with an electric stirrer, a thermometer and a dropping funnel, add 55g sodium cyanide, 55mL methanol and a small amount of phase transfer catalyst quaternary ammonium salt, stir and heat When the temperature reaches 55°C, 100g of homemade methyl chloroacetate was added dropwise, and the dripping was completed in 15 minutes. The reaction was kept for 3 hours. After the reaction solution was filtered, the solvent methanol was first distilled under normal pressure, and then distilled under reduced pressure. Collect 88~90°C/2.666 The fraction of kPa is the product methyl cyanoacetate.
![]()
Purpose
1. It is an intermediate for medicines, pigments, pesticides, etc. It is used to condense with formaldehyde to produce poly-2-methyl cyanoacrylate, which is then depolymerized to form a-methyl cyanoacrylate, which is then formulated into commonly known as 501 cyanoacrylate. 2.Organic synthetic raw materials and pharmaceutical dye intermediates, mainly used in the manufacture of adhesives, methyl cyanoacrylate, vitamin 6, etc.
