p-Aminoacetanilide p-Aminoacetanilide
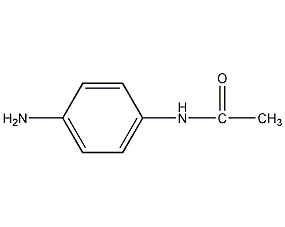
Structural formula
| Business number | 03FK |
|---|---|
| Molecular formula | C8H10N2O |
| Molecular weight | 150.18 |
| label |
4-Aminoacetylaniline, N-(4-aminophenyl)acetamide, N-acetyl p-phenylenediamine, Para-aminoacetanilide, N-(4-Aminophenyl)acetamide, N-Acetyl-p-phenylenediamine, 4-Aminoacetanilide, Fragrance |
Numbering system
CAS number:122-80-5
MDL number:MFCD00007853
EINECS number:204-576-6
RTECS number:AD8225000
BRN number:742888
PubChem number:24846417
Physical property data
1. Character: white or reddish crystal. Color deepens in air.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 162
5. Boiling point (ºC): 267
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V) : Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot water, ethanol, ether, slightly soluble in cold water .
Toxicological data
1. Skin/eye irritation: Rabbit eye standard Drez eye dye test: 100mg/24H is moderately irritating to the eyes.
2. Acute toxicity: Rat oral LD50: 2500mg/kg
Mouse oral LD50: 633mg/kg
3. Mutagenicity: Salmonella Mutation test system: 100ug/plate
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 44.76
2. Molar volume (cm3/mol): 124.7
3. Isotonic specific volume (90.2K): 336.8
4. Surface tension (dyne/cm): 53.0
5. Polarizability (10-24cm3 ): 17.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 55.1
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 139
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Poisonous. There is obvious allergic reaction in skin contact. Direct contact with skin and inhalation of dust should be avoided. Production personnel should wear protective equipment when operating.
Storage method
Seal, fill with nitrogen and store in a cool, dry place away from light.
Packed in iron drums lined with plastic bags, 25kg per drum. It should be stored in a cool, ventilated, dry place away from light and away from sunlight. Handle it with care when transporting, and protect it from heat and moisture. Store and transport according to regulations on toxic chemicals.
Synthesis method
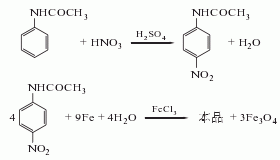
1. Acetanilide method: Nitration of acetic water (acetanilide) with mixed acid produces p-nitroacetanilide, which is then reduced to p-aminoacetanilide with iron powder. The reaction solution is neutralized, crystallized, and dried to obtain the finished product. (1) Nitrification Add 675kg of concentrated sulfuric acid (98%) into the kettle, stir, add 225kg of acetanilide (99%) at 20-25°C within 2-2.5 hours, and complete the addition until it is completely dissolved. Cool to 7°C and add mixed acid (made up of 63kg water, 60kg 98% sulfuric acid and 107kg 96% nitric acid) dropwise within 20 hours at 4-7°C. After the dropwise addition is completed, dilute it in 4000L ice water and let it stand for 1 hour. The waste acid in the upper layer is separated by siphon, and the material in the lower layer is filtered and washed with water until neutral to obtain p-nitroacetanilide. (2) Reduction: Add 700kg of water to the reduction barrel, stir, add 110kg of iron powder and 4kg of acetic acid (98%), raise the temperature to 80°C, add half of the above nitro substance to 2-2.5kg, and control the temperature at 72-75 ℃. After adding, keep warm for 1 hour and let stand for 1 hour. Suck the upper layer liquid into the neutralization kettle, neutralize it to pH=8 with soda ash (about 4kg) at 70-75°C, and add a small amount of alkali sulfide to remove iron ions. Let it stand for 1 hour, suck the supernatant liquid into the scraper crystallization tank, and cool it to 18°C. Centrifugally filter and dry to obtain about 200kg of p-aminoacetanilide, with a total yield of 80%.
2. Para-nitroaniline method: Put para-nitroaniline into the reaction kettle, mix it with acetic acid and acetic anhydride, heat for 4-5 hours, add water to filter out the crystals, wash and filter; then Add water, iron powder and acetic acid to the washed p-nitroacetanilide, heat, stir and keep for 4 hours, decolorize, filter to remove iron oxide, the filtrate will crystallize by cold, filter, and recrystallize with ethanol to obtain the finished product. Raw material consumption quota: acetanilide (98%) 1210kg/t, sulfuric acid (98%) 4000kg/t, nitric acid (95%) 577kg/t.

Purpose
Dye intermediates. Mainly used to prepare disperse dyes, acid dyes, such as disperse yellow G, disperse blue H3R, disperse blue 5R, direct acid-resistant vermilion 4BS, direct light-fast maroon, acid-resistant fuchsin 6B, reactive blue AG, black salt ANB and neutral brilliant blue GV et al.
