p-Xylene p-Xylene
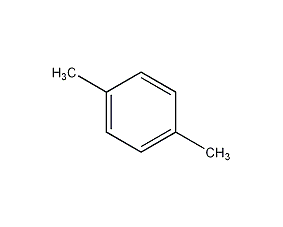

Structural formula
| Business number | 02TE |
|---|---|
| Molecular formula | C8H10 |
| Molecular weight | 106.16 |
| label |
1,4-xylene, 1,4-Dimethylbenzene, p-Xylol, aromatic compounds |
Numbering system
CAS number:106-42-3
MDL number:MFCD00008556
EINECS number:203-396-5
RTECS number:ZE2625000
BRN number:1901563
PubChem number:24890185
Physical property data
1. Properties: Colorless transparent liquid with an odor similar to toluene. [1]
2. Melting point (℃): 13.3[2]
3. Boiling point (℃): 138.4 [3]
4. Relative density (water = 1): 0.86[4]
5. Relative vapor density (Air=1): 3.66[5]
6. Saturated vapor pressure (kPa): 1.16 (25℃)[6]
7. Heat of combustion (kJ/mol): -4559.8[7]
8. Critical temperature (℃): 359[8]
9. Critical pressure (MPa): 3.51[9]
10. Octanol/water partition coefficient: 3.15[ 10]
11. Flash point (℃): 25 (CC) [11]
12. Ignition temperature (℃) :528[12]
13. Explosion upper limit (%): 7[13]
14. Explosion lower limit (%) %): 1.1[14]
15. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol, ether, chloroform, acetone, and benzene. [15]
16. Viscosity (mPa·s, 25ºC): 0.603
17. Heat of evaporation (KJ/mol, 101.3kPa): 36.00
18. Heat of evaporation (KJ/mol, 1.16kPa): 42.40
19. Heat of fusion (KJ/mol, 101.3kPa): 17.12
20 . Heat of formation (KJ/mol, 25ºC, gas): 17.96
21. Heat of formation (KJ/mol, 25ºC, liquid): -24.43
22. Heat of combustion (KJ /mol, 25ºC, gas): 4598.32
23. Heat of combustion (KJ/mol, 25ºC, liquid): 4555.90
24. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.20
25. Thermal conductivity (W/(m·K), 30≤t≤125 ºC): (0.1375~2.302)×10-4 t
26. Relative density (20℃, 4℃): 0.8610
27. Refractive index at room temperature (n20): 1.4958
p>
28. Critical density (g·cm-3): 0.281
29. Critical volume (cm3·mol-1): 378
30. Critical compression factor: 0.259
31. Eccentricity factor: 0.346
32. Lennard-Jones parameters (A): 15.83
33. Lennard-Jones parameter (K): 1623.1
34. Solubility parameter (J·cm-3)0.5: 17.838
35. van der Waals area (cm2·mol-1): 8.840×109
36. van der Waals volume (cm3·mol-1): 70.660
37. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4595.27
38. Gas phase standard claimed heat (enthalpy) (kJ·mol– 1): 18.06
39. Gas phase standard entropy (J·mol-1·K-1): 352.34
40. Gas phase standard formation free energy (kJ·mol-1): 121.5
41. Gas phase standard hot melt (J·mol-1Lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Petroleum xylene and coal tar xylene contain considerable amounts of paraxylene. Since the boiling point difference between para-xylene and m-xylene is only 0.75°C, the distillation separation method cannot be used. The methods currently being researched and developed at home and abroad are low-temperature crystallization separation method, adsorption separation method and complex separation method. The low-temperature crystallization separation method uses the difference in melting points of xylene isomers to separate. The main method is cryogenic step-by-step crystallization. The process technology is mature and currently dominates xylene separation. However, the equipment of this method is huge, paraxylene is limited by the eutectic point, and the recovery rate is low, only 60-70%. The adsorption separation method is a new method developed in the 1970s. This method has less investment than the cryogenic crystallization method, lower total production costs, high p-xylene yield, and high purity. It may replace the cryogenic crystallization method. 2. The raw material toluene undergoes an alkylation reaction in the transalkylation reactor to generate xylene and benzene. Mixed xylene is used in the isomerization reactor to isomerize part of m-xylene to generate p-xylene. After the light fraction is removed in the stabilization tower, the reactant is mixed with the xylene from the alkyl transfer section and enters the C9 removal tower. Mixed xylenes with higher paraxylene content are obtained at the top of the tower, and the tower kettle contains components above C9. The mixed xylene obtained from the top of the stabilizing tower enters the adsorption separation section, where non-molecular sieve solid adsorbent is used to adsorb para-xylene and desorb to obtain a para-xylene product with a purity of up to 99.9%, and at the same time, m-xylene is produced as a by-product. In addition, there is also a hydrogen fluoride-boron trifluoride extraction method.
2. The mixed xylene obtained from petroleum is separated and refined, and then fractionated and crystallized through efficient distillation. Solvents used include methanol, ethanol, isopropyl alcohol, acetone, butanol, toluene, pentane or pentene, etc. To prepare chromatographically pure paraxylene, nitrogen can be used as the carrier gas, and the crude product or mixed xylene can be injected into a preparative gas chromatograph equipped with an OV-1*-Bentone34*/white diatomite carrier stationary phase column, and then separated and collected. The p-xylene component is then put into a glass ampoule and sealed.
*: A polymeric silicone oil produced in the United States for use as a gas chromatography stationary phase.
*: A brand of organic bentonite produced in the United States.
Purpose
1. Used as a raw material for the production of polyester fibers and resins, coatings, dyes and pesticides. It is also used as a standard material and solvent for chromatographic analysis and in organic synthesis.
2. Used to produce terephthalic acid, and then to produce polyester resins such as ethylene glycol terephthalate and butylene glycol ester. Polyester resin is the raw material for the production of polyester fibers, polyester sheets, and polyester hollow containers. Polyester fiber is currently the largest synthetic fiber in my country. It is also used as raw material for coatings, dyes and pesticides.
3. As a raw material for synthetic polyester fibers, resins, coatings, dyes and pesticides. [31]
