2-(2-Butoxyethoxy)ethanol 2-(2-Butoxyethoxy)ethanol
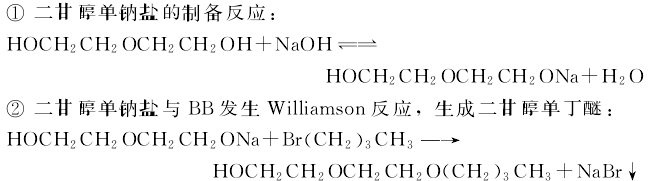

Structural formula
| Business number | 035G |
|---|---|
| Molecular formula | C8H18O3 |
| Molecular weight | 162.23 |
| label |
Diethylene glycol monobutyl ether, Diethylene glycol monobutyl ether, Dihydroxyethyl butyl ether, Butyl carbitol, 2-(2-butoxyethoxy)ethanol, Diethylene glycol butyl ether, Diethylene glycol monomethyl ether, 2-Hydroxyethyl ether, C4H9OC2H4OC2H4OH, thinner, stabilizer, Stabilizer for latex paint, Evaporation inhibitors for aircraft coatings, Surface processing improver for high temperature baked enamels |
Numbering system
CAS number:112-34-5
MDL number:MFCD00002881
EINECS number:203-961-6
RTECS number:KJ9100000
BRN number:1739225
PubChem number:24878239
Physical property data
1. Properties: Colorless liquid, slightly fragrant.
2. Density (g/mL, 20/20ºC): 0.9536
3. Melting point (ºC): -68
4. Boiling point (ºC, Normal pressure): 231
5. Refractive index (20ºC): 1.4316
6. Viscosity (mPa·s, 20ºC): 6.49
7. Flash Point (ºC, open): 93
8. Flash point (ºC, closed): 78
9. Fire point (ºC): 227
10. Heat of evaporation (KJ/mol, b.p.): 41.9
11. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 2.29
12. Vapor pressure (kPa , 20ºC): 0.001
13. Vapor pressure (kPa, 109ºC): 1.33
14. Vapor pressure (kPa, 145ºC): 6.67
15. Volume expansion coefficient (K-1, 10~30ºC): 0.00087
16. Explosion upper limit (%, V/V): 6.2
17. Lower explosion limit (%, V/V): 0.9
18. Solubility: Miscible with water, can dissolve grease, dyes, natural resins, nitrocellulose, etc. Polyvinyl acetate is partially soluble, but cellulose acetate, polystyrene, and polymethylmethacrylate are not.
19. Relative density (20℃, 4℃): 0.9553
20. Refractive index at room temperature (n20): 1.4306
21. Normal temperature refractive index (n25): 1.427027
22. Liquid phase standard hot melt (J·mol-1·K-1): 355.9
Toxicological data
1. Irritation: Rabbit transocular Drez eye dye test: 20mg is severely irritating.
Rabbit transdermal Derez eye dye test: 20mg/24H Moderate irritation.
2. Acute toxicity: Rat oral LD50: 6560mg/kg; Mouse oral LD50: 2400mg/kg;
Mouse abdominal LD50: 850mg/kg; Rabbit Oral LD50: 2200mg/kg;
Rabbit transdermal LD50: 2700mg/kg; Guinea pig oral LD50: 2gm/kg;
3. Other multiple doses: Rabbit oral TDLO : 83gm/kg/13W-i; Rat passing TDLO: 109GM/KG/6W-I;
Rat inhaled TCLO: 5mg/m3/24H/17W-C
<p
4. It is slightly toxic and can cause moderate irritation to the eyes and short-term corneal damage.
Ecological data
Has slight harm to water bodies.
Molecular structure data
1. Molar refractive index: 44.13
2. Molar volume (cm3/mol): 170.8
3. Isotonic specific volume (90.2K ): 406.4
4. Surface tension (dyne/cm): 32.0
5. Polarizability (10-24cm3): 17.49
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 38.7
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 66.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxides.
Colorless liquid, slightly fragrant, non-toxic. Easily soluble in alcohol and ether, soluble in water and oil. Is a flammable substance. Non-corrosive to metals. It has the chemical properties of alcohol and ether.
Storage method
Stored sealed in a cool, dry place. Make sure the workspace has good ventilation. Keep away from sources of fire. Store away from oxidizing agents.
Synthesis method
1. First add butanol and boron trifluoride ether solution into the reactor, heat to 75-80°C, introduce ethylene oxide, and continue to react at 75-80°C for 2-3 hours after adding ethylene oxide. . After the reaction is completed, neutralize it with 5% sodium butoxide to pH 8 and send it to the distillation tower for distillation. The low boiling materials are butanol and ethylene glycol monobutyl ether, and the high boiling materials are diethylene glycol monobutyl ether. For crude ether, continue distillation and collect the fraction at 230.5~231.5°C under normal pressure as the finished product.
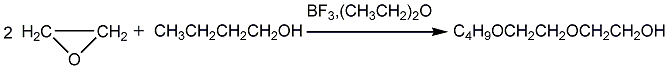
2. Industrial production can produce ethylene glycol In the monobutyl ester process, ethylene glycol monobutyl ether continues to react with ethylene oxide to obtain diethylene glycol monobutyl ether as a by-product. 
Refining method: anhydrous potassium carbonate or sodium sulfate After drying, fractionate under reduced pressure. If there is peroxide, it can be removed by refluxing stannous chloride. Peroxides can also be removed by passing butyl diglyme through a column containing activated alumina under slight pressure.
3.Diethylene glycol (DEG) is a by-product produced during the hydration of n-butyl bromide (BB) and ethylene oxide to produce ethylene glycol. ) as the initial raw material, the new process for synthesizing diethylene glycol monobutyl ether is to add a certain amount of toluene, DEG, and solid alkali in a dry four-neck bottle with a water separator and a stirrer in sequence, and slowly raise the temperature to reflux while stirring. When the amount of water separation is close to the theoretical amount, add a certain amount of BB from the dropping funnel. After the dripping is completed, reflux for a certain time and then cool down, filter out the NaBR solid precipitate, and evaporate the toluene under normal pressure; then fractionate under reduced pressure at 40KPa, and collect 120~130°C separately. (Diethylene glycol monobutyl ether) and 130~145℃ (DEG) fractions. The product diethylene glycol monobutyl ether is a colorless and transparent liquid. Raw material ratio: DEG: solid alkali: toluene: n-butyl bromide = 5:1.4:6:1 (molar ratio), etherification reaction time is 0.75h. The synthesis reaction is as follows. 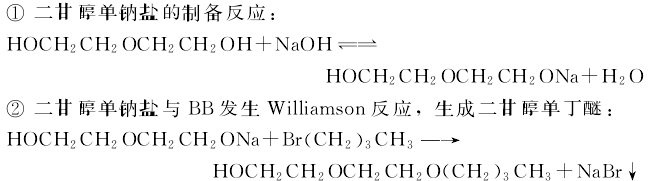
Purpose
Used as a solvent for nitrocellulose, varnish, printing ink, stamp pad ink, oils, resins, etc. Thinner for adhesives, stabilizer for latex paint, evaporation inhibitor for aircraft paint, and surface processing improver for high-temperature baked enamel paint. Also used as a solvent to separate propyne from acetylene.
.gif” alt=”” />
Purpose
Used as a solvent for nitrocellulose, varnish, printing ink, stamp pad ink, oils, resins, etc. Thinner for adhesives, stabilizer for latex paint, evaporation inhibitor for aircraft paint, and surface processing improver for high-temperature baked enamel paint. Also used as a solvent to separate propyne from acetylene.
