Paraldehyde Paraldehyde
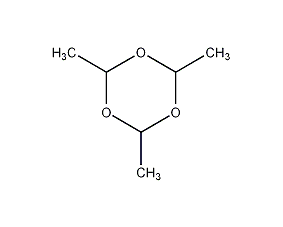

Structural formula
| Business number | 03GX |
|---|---|
| Molecular formula | C6H12O3 |
| Molecular weight | 132.16 |
| label |
Acetaldehyde, 2,4,6-Trimethyl-1,3,5-triethylene oxide, Polytriacetaldehyde, paraldehyde, aldehyde, Acetaldehyde trimmer, paracetaldehyde, thinner, heterocyclic compounds, aldehyde solvent |
Numbering system
CAS number:123-63-7
MDL number:MFCD00036208
EINECS number:204-639-8
RTECS number:YK0525000
BRN number:80142
PubChem number:24898662
Physical property data
1. Properties: Colorless oily liquid with aromatic odor. [1]
2. Melting point (ºC): 12.6[2]
3. Boiling point (ºC): 124.5 [3]
4. Relative density (water = 1): 0.99[4]
5. Relative vapor density (Air=1): 4.5[5]
6. Saturated vapor pressure (kPa): 3.36 (20ºC)[6]
7. Heat of combustion (kJ/mol): -3119.4[7]
8. Critical temperature: 290.0[8]
9. Critical pressure (MPa): 3.5[9]
10. Octanol/water partition coefficient: 0.67[10] sup>
11. Flash point (ºC): 35.5 (OC) [11]
12. Ignition temperature (ºC): 235[12]
13. Explosion upper limit (%): 17[13]
14. Explosion lower limit (%): 1.3 [14]
15. Solubility: soluble in water and miscible in most organic solvents. [15]
16. Refractive index at room temperature (n25): 1.41049
17. Refractive index at room temperature (n20): 1.8759117.2
18. Solubility parameter (J·cm-3)0.5 sup>: 17.644
19. van der Waals area (cm2·mol-1): 9.870×109
20. van der Waals volume (cm3·mol-1): 72.450
21. Gas-phase standard combustion Heat (enthalpy) (kJ·mol-1): -3444.2
22. The gas phase standard claims heat (enthalpy) (kJ·mol-1 ): -631.8
23. Gas phase standard entropy (J·mol-1·K-1): 374.47
24. Gas phase standard free energy of formation (kJ·mol-1): -407.6
25. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1 ): -3402.8
26. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -673.2
27 .Liquid phase standard entropy (J·mol-1·K-1): 289.37
28. Liquid phase standard free energy of formation (kJ· mol-1): -424.0
29. Liquid phase standard hot melt (J·mol-1·K-1):258.8
Toxicological data
1. Acute toxicity[16] LD50: 1530mg/kg (rat oral); 14000mg/kg (rabbit oralSkin)
2. Irritation [17]
Rabbit transdermal: 500mg, mild irritation (open Irritation test)
Rabbit eye: 5mg, severe irritation.
3. Mutagenicity [18] Cytogenetic analysis: Saccharomyces cerevisiae 50mmol/tube.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[19] MITI-Ⅱ test , the initial concentration is 28.9ppm, the sludge concentration is 100ppm, and the degradation is 12% after 4 weeks.
3. Non-biodegradability[20] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 45h (theoretical).
Molecular structure data
1. Molar refractive index: 30.47
2. Molar volume (cm3/mol): 88.6
3. Isotonic specific volume (90.2K ): 241.4
4. Surface tension (dyne/cm): 54.9
5. Polarizability (10-24cm3): 12.08
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 63.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[21] Stable
2. Incompatible substances[22] Strong oxidants, alkalis, hydrocyanic acid, plastics, rubber
3. Conditions to avoid contact[23] Heating
4. Hazards of aggregation[24] No aggregation
Storage method
Storage Precautions[25] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C and the container should be kept sealed. They should be stored separately from oxidants, acids, alkalis, etc. and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Acetaldehyde can polymerize to form paraldehyde, metaldehyde, etc. during storage. In industrial production, inorganic acids such as sulfuric acid are used as catalysts to polymerize acetaldehyde.
Purpose
1. Paraldehyde is easily decomposed into acetaldehyde, which can be used as a stable form of acetaldehyde to facilitate the storage and transportation of acetaldehyde. About 50% of paraldehyde is used in the field of pesticides in Japan, followed by incense, medicine, solvents and thinners for coatings, etc.
2. Used as a solvent, as well as in organic synthesis, rubber accelerator and antioxidant manufacturing, etc. [26]
