Ethylene Glycol Dibutyl Ether Ethylene Glycol Dibutyl Ether
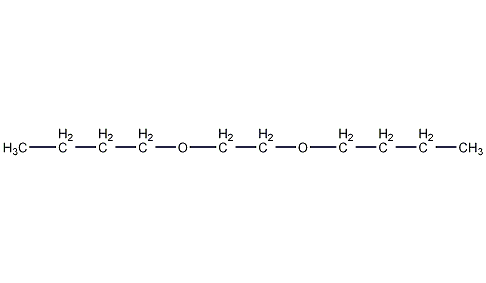
Structural formula
| Business number | 035V |
|---|---|
| Molecular formula | C10H22O2 |
| Molecular weight | 174.28 |
| label |
1,2-dibutoxyethane, Ethylene glycol di-n-butyl ether, 5-Acetylbenzothiophene, 1,2-Dibutoxyethane, Dibutyl Cellosolve, Dibutyl Glycol, Dispersant, inert solvent |
Numbering system
CAS number:112-48-1
MDL number:MFCD00048785
EINECS number:203-976-8
RTECS number:KH9450000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: colorless liquid with slight ether smell.
2. Boiling point (ºC, 101.3kPa): 203.1
3. Melting point (ºC): -69.1
4. Relative density (g/mL, 20/4ºC): 0.8365
5. Refractive index (20ºC): 1.4131
6. Viscosity (mPa·s, 20ºC): 1.34
7. Flash point (ºC, open): 85
8. Heat of evaporation (KJ/mol): 47.73
9. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure ): 2.01
10. Vapor pressure (kPa, 20ºC): 0.012
11. Solubility: compatible with acetone, ethanol, 1,2-dichloroethane, ethyl acetate Miscible with ester, toluene, ether, heptane, castor oil, pine oil, isopropyl ether, etc. It dissolves 0.2% in water at 20℃; water dissolves 0.6% in ethylene glycol dibutyl ether. It can form a binary azeotropic mixture with water, with an azeotropic point of 99.1°C, and the azeotropic distillate contains 76.8% water.
12. Refractive index at room temperature (n25): 1.40623
Toxicological data
The vapor pressure is low at room temperature, so the possibility of poisoning by inhaling its vapor is small. Less toxic than ethylene glycol monobutyl ether. However, chronic poisoning can cause damage to the kidneys, so high temperatures and spray use should be avoided.
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 51.86
2. Molar volume (cm3/mol): 206.3
3. Isotonic specific volume (90.2K ): 469.3
4. Surface tension (dyne/cm): 26.7
5. Polarizability (10-24cm3): 20.56
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 9
5. Tautomers.Mass: None
6. Topological molecule polar surface area 18.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 64.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain stereocenters of atoms: 0
13. The number of determined stereocenters of chemical bonds: 0
14. The number of uncertain stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
It is a flammable liquid. Use carbon dioxide to extinguish fires. Non-corrosive to metals. During storage, peroxide can be generated by the action of oxygen in the air, and light can promote the generation of peroxide.
Chemical properties: Stable to bases, ether bonds are broken when reacting with strong acids. Peroxide can be formed under the action of oxygen in the air.
Storage method
It should be stored sealed and protected from light, and reducing agents such as ferrous sulfate, stannous chloride, and sodium sulfite should be added to control the generation of peroxides. Can be stored in iron, mild steel, copper or aluminum containers.
Synthesis method
Refining method: The test of peroxide is the same as that of ether. The method for removing peroxide and moisture is the same as for ethylene glycol diethyl ether. Finally fractionated and refined.
Purpose
It is used as a dispersant, and is also used as an inert solvent in the extraction of fatty acids from dilute fatty acid solutions, the separation and refining of alkyl phosphoric acids, and the extraction of uranium ore.
