Chloromethylmethyl ether Chloromethylmethyl ether
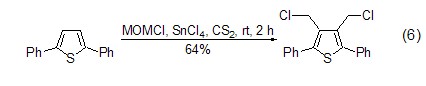
![]()
Structural formula
| Business number | 02V2 |
|---|---|
| Molecular formula | C2H5ClO |
| Molecular weight | 80.51 |
| label |
Methyl chloride, Chloromethyl methyl ether, Chlorodimethyl ether, Methyl chloromethyl ether, Methyl chloride, Chloromethoxymethane, Methoxymethyl chloride, Methyl chloromethyl ether, MOMCl, Aliphatic alcohols, ethers and their derivatives |
Numbering system
CAS number:107-30-2
MDL number:MFCD00000885
EINECS number:203-480-1
RTECS number:KN6650000
BRN number:None
PubChem number:24846524
Physical property data
1. Properties: colorless liquid with special smell. [14]
2. Melting point (℃): 7.5[15]
3. Boiling point (℃): 162 [16]
4. Relative density (water = 1): 1.07[17]
5. Relative vapor density (Air=1): 4.37[18]
6. Saturated vapor pressure (kPa): 0.35 (20℃)[19]
7. Heat of combustion (KJ/mol): -3754[20]
8. Critical temperature (℃): 385.7[21]
9. Critical pressure (MPa): 3.91[22]
10. Octanol/water partition coefficient: 3.33[ 23]
11. Flash point (℃): 49; 60 (OC) [24]
12. Ignition temperature ( ℃): 595[25]
13. Explosion upper limit (%): 6.7[26]
14. Explosion Lower limit (%): 1.3[27]
15. Solubility: used in organic synthesis, preparation of dye intermediates, and as a solvent. [28]
Toxicological data
1.Acute toxicity[29]
LD50: 500mg/kg (rat oral)
LC50: 179.8mg/m3 (rat inhalation, 7h); 1030mg/m3 (mouse inhalation, 2h)
2. Irritation No data available
3. Subacute and chronic toxicity[30] Rabbits exposed to a concentration of 0.003~0.008mg/L, 5 hours a day for 3 months, caused slow weight gain, pneumonia, tissue respiratory depression and reduced liver glycogen content.
4. Mutagenicity[31] DNA inhibition: human lymphocytes 5ml/L. Tumor transformation: hamster embryo 10mg/L.
5. Carcinogenicity[32] IARC Carcinogenicity Comment: G1, confirmed human carcinogen.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[33]
Aerobic biodegradation (h): 168~672
Anaerobic biodegradation(h): 672~2688
3. Non-biodegradability[34]
Photooxidation half-life in air (h): 22.7~227
First-order hydrolysis half-life (h): 0.033
Molecular structure data
1. Molar refractive index: 17.90
2. Molar volume (cm3/mol): 79.2
3. Isotonic specific volume (90.2K ): 169.8
4. Surface tension (dyne/cm): 21.0
5. Polarizability: 7.09
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 10
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[35] Stable
2. Incompatible substances[36] Strong oxidants, strong bases, acids
3. Conditions to avoid contact[37] Humid air
4. Polymerization hazard[38] No polymerization
5. Decomposition products[39] Hydrogen chloride, formaldehyde gas
Storage method
Storage Precautions[40] Usually products contain stabilizers. Store in a cool, well-ventilated special warehouse, and implement the system of “two people to send and receive, and two people to keep”. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, alkalis, and food chemicals, and avoid mixed storage. Not suitable for long-term storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. In the presence of calcium chloride, hydrogen chloride gas is passed into a mixture of methanol and formaldehyde, and aldol condensation and addition reactions occur at slightly higher than room temperature. Add industrial hydrochloric acid to the hydrogen chloride generating kettle (the by-product hydrogen chloride tail gas can also be used), add sulfuric acid (92%) dropwise at 70°C, and pass the generated hydrogen chloride gas into a reaction kettle filled with methanol and formaldehyde solutions for chlorination. The reaction temperature was controlled below 40°C, and the weight ratio of methanol (98%) and formaldehyde (37%) was 1:2.39. When the reaction kettle is saturated with hydrogen chloride, the chlorination reaction is completed. Then let it stand for 1 hour and separate into layers. The upper layer is the synthesized chloromethyl ether and the lower layer is the acid layer. Finally, the chloromethyl ether and the acid layer are separated. The yield of chloromethyl ether is 78%. Raw material consumption quota: methanol (98%) 0.5t/t, formaldehyde (37%) 1.2t/t, sulfuric acid (92%) 3.5t/t, hydrochloric acid (33%) 2.6t/t.
![]()
2. Dimethyl formaldehyde Acetals are prepared in the presence of acetyl chloride[1].
Purpose
1. Used as a solvent, active organic intermediate, and chloromethylating agent. It is mainly used in the production of anion exchange resins and also in the production of sulfadiazine drugs.
2. As a chloromethylating reagent, u can be used to introduce a chloromethyl group on the aromatic ring. Methoxymethylation reagent can be used to introduce methoxymethyl groups on alcohol (phenol) radicals and β-keto acid ester molecules. It is also a protective reagent for alcohols (phenols) and carboxylic acids.
3. (Chloromethyl)methyl ether (MOMCl) has two main uses in organic synthesis. On the one hand, it is used as a protecting group for alcohol, phenol, carboxylic acid and amide functional groups; on the other hand, it is used as a protective group for alcohol, phenol, carboxylic acid and amide functional groups; Aspects are used as chloromethylation reagents.
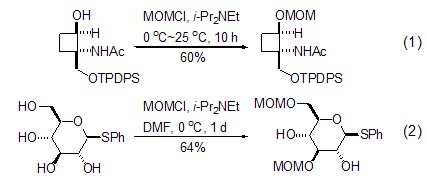
(Chloromethyl)methyl ether is one of the most important protective groups for alcohols and plays an important role in organic synthesis. Its basic advantages are that it is easy to protect and has good yield; it can be deprotected under multiple conditions and has a wide range of applications [2]. The reaction with alcohol is the most frequently used function of (chloromethyl)methyl ether, and the reaction conditions are very mild. Generally, it needs to be carried out in the presence of a base. The most commonly used base isn-Pr2NEt (Formula 1)[3~5]. In the presence of multiple hydroxyl groups, a high degree of regioselectivity can be achieved by appropriately selecting conditions (Formula 2)[4,5].
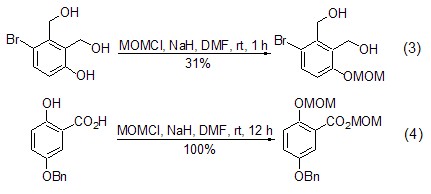
Phenolic hydroxyl and (chloromethyl ) methyl ether reacts more easily [6,7]. When the substrate molecule contains both alcoholic and phenolic hydroxyl groups, by controlling the reaction conditions, the reaction occurs preferentially on the phenolic hydroxyl group (Formula 3)[8]. When the substrate molecule contains both phenolic hydroxyl and carboxylic acid, the acid can be converted into the corresponding ester while converting the phenol into the ether (Formula 4)[9].
The activated amide and ( Chloromethyl)methyl ether can be easily converted into the corresponding N-MOM derivative (Formula 5) [10,11] .
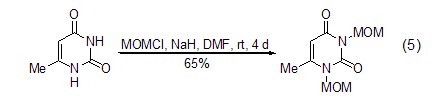
Under the action of Lewis acid reagent , (Chloromethyl)methyl ether can be used as an excellent one-carbon alkylation reagent. It can react with chloromethyl on the aromatic ring very conveniently and with high yield, and introduce chloromethyl functional groups into the substrate molecules (formula 6)[12,13].
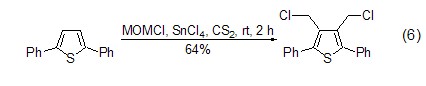
4. As chloromethylation agent. [41]
can be easily converted into the corresponding N-MOM derivatives (Formula 5)[10,11].
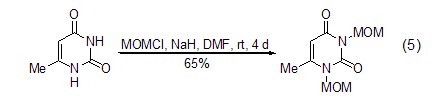
Under the action of Lewis acid reagent , (Chloromethyl)methyl ether can be used as an excellent one-carbon alkylation reagent. It can react with chloromethyl on the aromatic ring very conveniently and with high yield, and introduce chloromethyl functional groups into the substrate molecules (formula 6)[12,13].
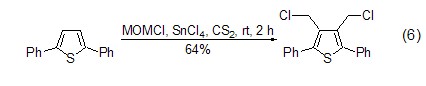
4. As chloromethylation agent. [41]
