2,4,4-Trimethyl-2-pentene 2,4,4-Trimethyl-2-pentene
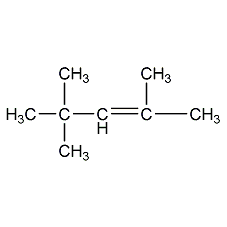

Structural formula
| Business number | 02V7 |
|---|---|
| Molecular formula | C8H16 |
| Molecular weight | 112.21 |
| label |
diisobutylene, β-Diisobutylene, (CH3)3CCH=C(CH3)2 |
Numbering system
CAS number:107-40-4
MDL number:MFCD00008902
EINECS number:203-488-5
RTECS number:None
BRN number:1719492
PubChem number:24889687
Physical property data
1. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5329.84
2. Density (g/mL, 25℃): 0.72
3. Relative vapor density (g/mL, air=1): >1
4. Melting point (ºC): -106
5. Boiling point ( ºC, normal pressure): 104
6. Solubility parameter (J·cm-3)0.5: 14.980
7. Refractive index (D20): 1.416
8. van der Waals area (cm2·mol-1): 1.229×1010
9. van der Waals volume (cm3·mol– 1): 85.160
10. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -104.89
11. Vapor pressure (mmHg, 37.7ºC): 83
12. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -5292.34
13. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -142.38
14. Liquid phase standard entropy (J·mol-1 ·K-1): 311.7
15. Liquid phase standard free energy of formation (kJ·mol-1): 89.81
16. Liquid phase standard hot melt (J·mol-1·K-1): 239.7
17. Explosion Upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Undetermined
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 38.97
2. Molar volume (cm3/mol): 154.0
3. Isotonic specific volume (90.2K ): 328.9
4. Surface tension (dyne/cm): 20.7
5. Polarizability (10-24cm3): 15.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 87.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain atomic stereocenterNumber of stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
None
Purpose
Used for preparing spices
