2-Ethyl-2-methyl-1,3-dioxolane 2-Ethyl-2-methyl-1,3-dioxolane
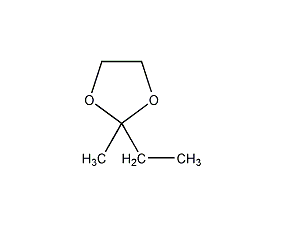
Structural formula
| Business number | 03K4 |
|---|---|
| Molecular formula | C6H12O2 |
| Molecular weight | 116.18 |
| label |
2-Ethyl-2-methyl-1,3-dioxolane, 2-ethyl-2-methyl-1,3-dioxin, 2-Ethyl-2-methyl-1,3-dioxolane, 2-ethyl-2-methyl-1,3-dioxopentane, 2-ethyl-2-methyl-1,3-dioxolane, MED, 2-Butanone ethylene acetal,, Alicyclic compounds |
Numbering system
CAS number:126-39-6
MDL number:MFCD00014108
EINECS number:204-785-2
RTECS number:JH7700000
BRN number:None
PubChem number:24860893
Physical property data
1. Boiling point (oC): 116-117
2. Solubility: soluble in most organic solvents, usually in CH2Cl2 or used under solvent-free conditions.
Toxicological data
1. Skin/Eye Irritation Data
Rabbit Eye Contact: Mild Reaction to 500mg/24H
Rabbit Skin Contact: Mild Reaction to 500mg/24H
2. Acute toxicity data
Rat oral LD50: 2380 uL/kg
Rat inhalation LCLo: 4000 ppm/4H
Rabbit skin LD50: 10mL/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 30.98
2. Molar volume (cm3/mol): 126.7
3. Isotonic specific volume (90.2K ): 297.1
4. Surface tension (dyne/cm): 30.1
5. Polarizability (10-24cm3): 12.28
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 18.5
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 74.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Highly flammable compound, relatively stable to air and moisture.
Storage method
None
Synthesis method
None
Purpose
2-Methyl-2-ethyl-1,3-dioxolane (MED) is mainly used as an acetalization exchange reagent in organic synthesis to transform the aldehyde and ketone functional groups in the substrate molecule. Become ethylene glycol�Acetal or ketal.
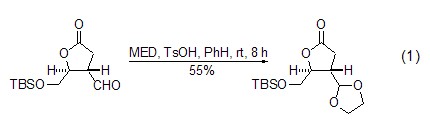
The acetal exchange reaction between MED and aldehydes occurs in the presence of acid catalyst to generate new ethylene glycol acetal. p-Toluenesulfonic acid or camphorsulfonic acid are the most commonly used catalysts for this reaction. Compared with directly using ethylene glycol to heat dehydration under acidic conditions, the reaction can be carried out under very mild conditions. In most cases, it can be completed by stirring at room temperature for several hours, which is very important for substrates with acid-sensitive functional groups (Formula 1)[1,2].
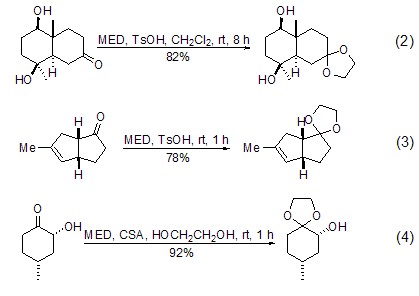
Under almost the same conditions, MED conveniently converts ketones into ethylene glycol ketals. CH2Cl2 or benzene are the most commonly used reaction solvents, and tertiary alcohols with high acid sensitivity are not significantly affected under these conditions (Formula 2)[3,4]. This reaction can also use excess MED as the reaction solvent (Formula 3)[5]. The recommended method is to use excess MED and add a small amount of ethylene glycol as a catalyst to obtain a better yield (Formula 4)[6,7].
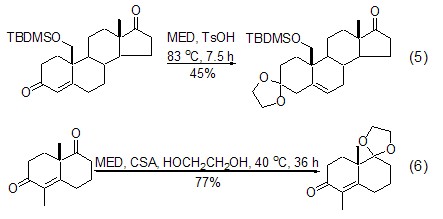
With dimethyl-1, Compared with 3-dioxolane, MED has higher regioselectivity in the reaction with substrates containing multiple ketone functional groups. The influence of the structure of the substrate on selectivity mainly comes from the influence of steric hindrance of the ketone functional group. Six-membered rings are preferred to five-membered rings (Formula 5)[8,9], and smaller steric hindrances are preferred to larger steric hindrances (Formula 6)[10,11].

