Oleoyl Chloride Oleoyl Chloride
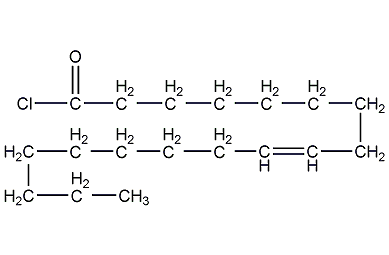

Structural formula
| Business number | 036M |
|---|---|
| Molecular formula | C18H33ClO |
| Molecular weight | 300.91 |
| label |
9-Octadecenoyl chloride, (Z)-9-Octadecenoylchloride, detergent, aliphatic compounds |
Numbering system
CAS number:112-77-6
MDL number:MFCD00134332
EINECS number:204-005-0
RTECS number:None
BRN number:1211748
PubChem number:24897935
Physical property data
1. Characteristics: Liquid.
2. Density (g/mL,20℃):0.912
3. Relative vapor density ( g/mL,Air=1): Undetermined
4. Melting point (ºC): -46
5. Boiling point (ºC,normal pressure): Undetermined
6. Boiling point (ºC,1.46Kpa):200
7. Refractive index:1.463
8. Flashpoint (ºC):110
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16.
4. Rotatable Number of chemical bonds: 15
5. Interchange Number of isomers:
6. Topological molecules Polar surface area (TPSA):17.1
7. Heavy atoms Quantity: 20
8. Surface charge :0
9. Complexity :236
10. Number of isotope atoms:0
11. Determine the number of atomic stereocenters:0
12. Uncertain number of atomic stereocenters:0
13. Determine the number of stereocenters of chemical bonds:1
14. Uncertain number of chemical bond stereocenters:0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
Originated from the reaction of oleic acid and phosphorus trichloride. Put oleic acid into the reaction pot, slowly add phosphorus trichloride while stirring, and control the feeding temperature at 25-33 ℃. After addition, heat up to55℃, insulate 4h and then separate the lower layer of phosphorous acid to obtain oleoyl chloride.
Purpose
Used as an intermediate in organic synthesis. Use oleoyl chloride to acylate 2-sulfo -4- aminoanisole to obtain a cleaning agent
18.0pt; mso-para-margin-left: -.01gd” align=left>10. Isotopes Number of atoms: 0
11. Determine the number of atomic stereocenters:0
12. Uncertain number of atomic stereocenters:0
13. Determine the number of stereocenters of chemical bonds:1
14. Uncertain number of chemical bond stereocenters:0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
None
Synthesis method
Originated from the reaction of oleic acid and phosphorus trichloride. Put oleic acid into the reaction pot, slowly add phosphorus trichloride while stirring, and control the feeding temperature at 25-33 ℃. After addition, heat up to55℃, insulate 4h and then separate the lower layer of phosphorous acid to obtain oleoyl chloride.
Purpose
Used as an intermediate in organic synthesis. Use oleoyl chloride to acylate 2-sulfo -4- aminoanisole to obtain a cleaning agent
AMILY: 宋体; mso-bidi-font-family: ‘Times New Roman’; mso-font-kerning: 1.0pt; mso-ansi-language: EN-US; mso-fareast-language: ZH-CN; mso-bidi -language: AR-SA”>Used as an intermediate in organic synthesis. Use oleoyl chloride to convert 2-sulfo-4- Cleaning agent can be obtained by acylation reaction of aminoanisole
