4-methylpyridine 4-Methylpyridine
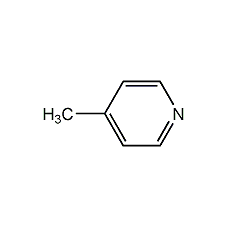
Structural formula
| Business number | 02Y6 |
|---|---|
| Molecular formula | C6H7N |
| Molecular weight | 93 |
| label |
γ-picolin, 4-picoline, γ-methylpyridine, 4-methylpyridine, 4-Picolin, picoline, γ-Picoline, 4-Methyl-pyridine, γ-Methylpyridine, p-Methylpyridine, 4-Picoline, Heterocyclic compounds |
Numbering system
CAS number:108-89-4
MDL number:MFCD00006440
EINECS number:203-626-4
RTECS number:UT5425000
BRN number:104586
PubChem number:24854384
Physical property data
1. Properties: colorless, flammable, volatile liquid. Has an unpleasant sweet taste. Impurities are brown.
2. Density (g/mL, 20/4℃): 0.9548
3. Relative vapor density (g/mL, air=1): 3.2
4. Melting point (ºC): 3.6
5. Boiling point (ºC, normal pressure): 144.9
6. Refractive index (20ºC): 1.5037
7. Viscosity (mPa·s, 20ºC): 0.94
8. Flash point (ºC, closed): 40
9. Flash point (ºC, open): 57
10. Heat of evaporation (KJ/mol, 25ºC): 45.35
11. Heat of fusion (KJ/mol): 11.58
12. Heat of formation (KJ /mol, liquid): 56.80
13. Heat of combustion (KJ/mol, 25ºC): 3420
14. Critical temperature (ºC): 372.5
15. Critical pressure (KPa): 4.66
16. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 1.70
17. pKa: 6.02
18. Body expansion coefficient (K-1, 25ºC): 0.000965
19. Solubility: soluble in water, ethanol and ether.
Toxicological data
1. Skin/eye irritation
Open irritation test: rabbit, skin contact: 480mg, severity of reaction: moderate.
Standard Draize test: Rabbit, eye contact: 20mg/24H, severity of reaction: moderate.
2. Acute toxicity: rat oral LD50: 440mg/kg; rat inhalation LCLo: 1000ppm/4H; rat abdominal LD50: 163mg/kg; mouse oral LD50: 350mg/ kg; mouse inhalation LC50: 4mg/m3; mouse abdominal LD50: 335mg/kg; rabbit skin contact LD50: 270μL/kg; guinea pig skin contact LDLo: 500mg/kg; wildOral LD50 for birds: 422mg/kg
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 29.16
2. Molar volume (cm3/mol): 98.9
3. Isotonic specific volume (90.2K ): 239.0
4. Surface tension (dyne/cm): 34.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 11.56
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 12.9
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 46.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants.
Chemical properties are similar to pyridine. It is alkaline and can form salts with inorganic acids and organic acids. It can also form addition compounds with inorganic salts, alkyl halides, etc. When hydrogenated, 4-methylpiperidine is formed. Isonicotinic acid is produced during oxidation. It condenses with benzaldehyde in the presence of dehydrating agent to form benzylidene derivatives.
2.Toxic, oral administration to rats is LD501.3g/kg. For protection methods, see Pyridine.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire, heat sources and anti-static. The storage temperature should not exceed 30℃. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
2. Packed in iron drums. 200kg per barrel. Packaging should be tight to prevent leakage. Store in a cool, dry place. Store and transport according to regulations on toxic chemicals.
Synthesis method
1. Produced by synthesis method and recovery from coal coking by-products. The reaction between acetaldehyde and ammonia mainly produces 2-methylpyridine and 4-methylpyridine. Preheat ammonia to 450°C, mix it with the gasified acetaldehyde, pass through a fluidized reaction tower equipped with an aluminum silicate catalyst preheated to 430°C, react at 410-430°C, and the generated gas is separated; condensed , put the obtained reaction solution into a dehydration pot, add 2% solid alkali, reflux and dehydrate at 92°C for 2 hours, let it stand for stratification; fractionate, collect the 142-144°C fraction to obtain 4-methylpyridine. The yield is 20% based on acetaldehyde (the 125-130°C fraction is 2-methylpyridine).
Raw material consumption quota: liquid ammonia 1174kg/t; acetaldehyde 3019kg/t.
![]()
2. Refining method: synthetic The product, which contains almost no 2-methylpyridine, is easily refined by distillation. Products derived from coal tar contain 2-methylpyridine and 2,6-lutidine. Calcium chloride solution and hydrochloric acid can be added to it, the resulting adduct crystals are washed with calcium chloride solution and decomposed with water vapor, then treated with salt water to remove soluble components, and then treated with potassium hydroxide solution to extract From the oil layer, 4-methylpyridine with a purity of 99.2% can be obtained. It can also be refined into oxalate. For example, heat 100 mL of 4-methylpyridine to 80°C, slowly add 110 g of anhydrous oxalic acid and 150 mL of boiling ethanol, filter after cooling, wash with a small amount of ethanol, then dissolve in a small amount of water, add excess 50% KOH and distill. The distillate is dried over solid potassium hydroxide and then distilled.
Purpose
It is used in the production of the drug isoniazid, the antidote drugs bisphosphonate and bisphosphonate, and is also used in the production of pesticides; dyes; rubber additives and synthetic resins. Used to prepare isonicotinic acid, isoniazid, pesticides, synthetic resins, rubber vulcanization accelerators, etc. It can also be used as a solvent.
