2-Methylpiperazine 2-Methylpiperazine
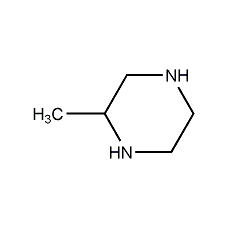

Structural formula
| Business number | 02YM |
|---|---|
| Molecular formula | C5H12N2 |
| Molecular weight | 100.16 |
| label |
(+/-)-2-Methylpiperazine, 2-Methyl-piperazin, MP, 2-Methyldiethylenediamine, (+/-)-2-Methylpiperazine, 2mprz, 2-Methylpiperazine/Lomefloxcin, 2-Methylpiperazine,99% |
Numbering system
CAS number:109-07-9
MDL number:MFCD00005954
EINECS number:203-644-2
RTECS number:TM1225020
BRN number:383566
PubChem number:24860387
Physical property data
1. Properties: colorless flaky crystals with piperazine odor.
2. Melting point (℃): 62
3. Flash point (℃): 63
4. Boiling point (℃): 135~155.5
p>
5. Solubility: Easily soluble in water, acetone, ethanol, chloroform and benzene. Aqueous solutions are alkaline.
Toxicological data
1. Acute toxicity: Rat oral LD50: 2030mg/kg
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.4
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 24.1
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 54
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides and moisture.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire, heat and water sources. The storage temperature should not exceed 30℃. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Use 1,2-propanediamine and ethanolamine as raw materials. 1,2-propanediamine and ethanolamine are made by passing ammonia gas into them and hydrogenating them under high temperature and pressure.
2. Use propylene oxide and ethylenediamine as raw materials. N-(β-hydroxypropyl)ethylenediamine is first made from propylene oxide and ethylenediamine and then closed-loop production.
�Way
Used to synthesize ofloxacin, levofloxacin, gatifloxacin and new quinolone antibacterial drug lomefloxacin.
