p-Cresyl acetate p-Cresyl acetate
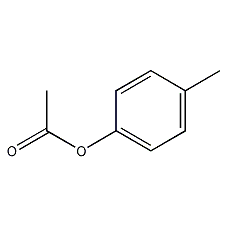

Structural formula
| Business number | 03T0 |
|---|---|
| Molecular formula | C9H10O2 |
| Molecular weight | 150.17 |
| label |
4-methylphenyl acetate, p-Creyl acetate, p-Tolyl acetate, acetic acid, 4-methylphenyl ester, food additives, Flavor enhancer |
Numbering system
CAS number:140-39-6
MDL number:MFCD00008703
EINECS number:205-413-1
RTECS number:AJ7570000
BRN number:1908125
PubChem number:24901197
Physical property data
1. Physical property data:
1. Properties: colorless liquid
2. Density (g/mL, 25/4℃): 1.048
3.Refractive index (nD20): 1.5163
4. Flash point (℃): 90
5. Boiling point (ºC): 210~213
6. Solubility: soluble in most organic solvents. Dissolve in 70% ethanol at 1:2 and 60% ethanol at 1:(9-12).
Toxicological data
II. Toxicological data:
1. Acute toxicity: Rabbit skin LD50: 2100 mg/kg
Rat oral LD50: 1900 mg/kg
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 42.41
2. Molar volume (cm3/mol): 143.3
3. Isotonic specific volume (90.2K): 346.4
4. Surface tension (dyne/cm): 34.1
5. Polarizability (10-24cm3): 16.81
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 135
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
2. Found in tobacco leaves.
3. Naturally found in cocoa extract.
Storage method
Store sealed in a dry and cool place.
Synthesis method
1. It is produced by direct esterification of phenol and acetic anhydride.
2. By �Cresol reacts with acetic anhydride in the presence of a small amount of sulfuric acid: after the reaction, it is washed with water and distilled to obtain the finished product.
Purpose
1. Suitable for narcissus, narcissus and large-flowered jasmine scents. It is also suitable for white orchid, lilac, lily of the valley, hyacinth, vanilla, and ylang-ylang. It has the effect of increasing the rich floral aroma. The dosage is generally less than 1%. It is sometimes used in nutty and nutty flavors in food flavors. middle.
2. Blending spices, blending ylang ylang, narcissus, cananga ylang, honeysuckle and other flavors. It has sufficient effect at concentrations below 1%. A small amount is used for food flavor.
3.GB 2760-96 stipulates that edible spices are allowed to be used. Mainly used for preparing banana, cherry, nut and mixed fruit flavors.
4. Used as flavoring agent
5. Used in baked goods and frozen dairy products.
