Phthalosulfonamide
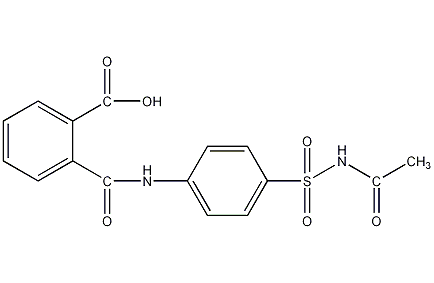

Structural formula
| Business number | 03N6 |
|---|---|
| Molecular formula | C16H14N2O6S |
| Molecular weight | 362.36 |
| label |
2-([(4-([Acetylamino]sulfonyl)phenyl)amino]carbonyl)benzoic acid, aromatic compounds |
Numbering system
CAS number:131-69-1
MDL number:MFCD00020275
EINECS number:205-035-7
RTECS number:None
BRN number:None
PubChem number:24898195
Physical property data
1. Characteristics: white crystalline powder, slightly odorous and slightly sour
2. Melting point (℃):186~202℃( Decomposition)
3. Solubility:Very slightly soluble in water, slightly soluble in ethanol, soluble in acetone, easily soluble in hydrogen oxide Sodium solution.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1、 Moore Refractive index: 87.97
2、 Moore Volume (m3/mol):245.0
3、 Isotonic specific volume (90.2K) :702.8
4、 Surface Tension (dyne/cm):67.6
5、 Polarizability (10-24cm3):34.87
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 4
6. Topological molecule polar surface area 138
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 619
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Introduction to production methods
Prepared from the acylation of p-aminobenzene sulfonacetamide and phthalic anhydride.
Purpose
Usage
<SPAN style="FONT-SIZE: 10pt; COLOR: #333333; FONT-FAMILY: Song�0.2K):702.8
4、 Surface Tension (dyne/cm):67.6
5、 Polarizability (10-24cm3):34.87
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: 4
6. Topological molecule polar surface area 138
7. Number of heavy atoms: 25
8. Surface charge: 0
9. Complexity: 619
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
None yet
Synthesis method
Introduction to production methods
Prepared from the acylation of p-aminobenzene sulfonacetamide and phthalic anhydride.
Purpose
Usage
This product is a sulfonamide drug. After oral administration, it slowly decomposes in the intestine to release sulfonamide acetyl, which produces intestinal antibacterial effects. It is clinically used for bacillary dysentery, enteritis and preparation before intestinal surgery. The efficacy is not as good as phthalosulfathiazole, but the absorption is stronger than phthalothiazolin.
��; mso-ascii-font-family: ‘Times New Roman’; mso-hansi-font-family: ‘Times New Roman'”>This product is a sulfonamide drug. After oral administration, it slowly decomposes in the intestine to release sulfonamide acetate. acyl to produce intestinal antibacterial effect. It is clinically used for bacillary dysentery, enteritis and preparation before intestinal surgery. The efficacy is not as good as phthalosulfathiazole, but the absorption is stronger than phthalosulfathiazole.
