Captan
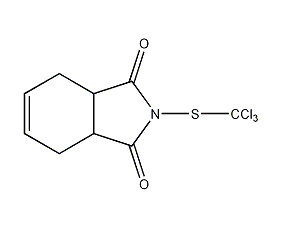

Structural formula
| Business number | 03NV |
|---|---|
| Molecular formula | C9H8Cl3NO2S |
| Molecular weight | 300.59 |
| label |
Kepton; N-trichloromethylthio-4-cyclohexene-1,2-dicarboximide; 1,2,3,6-tetrahydro-N-(trichloromethylthio)o-phenyl diimide, 1,2,3,6-Tetrahydro-N-(trichloromethylthio), N-(trichloro-methythio)-4-cyclohexene-1,2-dicarboximide, fungicides, bacteriostatic agent |
Numbering system
CAS number:133-06-2
MDL number:MFCD00041811
EINECS number:205-087-0
RTECS number:GW5075000
BRN number:234872
PubChem number:24868836
Physical property data
1. Properties: white crystal.
2. Density (g/mL, 20℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 178
5. Boiling point (ºC): Uncertain
6. Refractive index: Uncertain
7. Flash Point (ºC): Uncertain
8. Specific rotation (ºC): Uncertain
9. Autoignition point or ignition temperature (ºC) is uncertain
10. Vapor pressure (kPa, 25ºC): 1.33×10-2
11. Saturated vapor pressure (kPa, 60ºC): Uncertain
12. Heat of combustion (KJ/mol): Uncertain
13. Critical temperature (ºC): Uncertain
14. Critical pressure (KPa): Uncertain
15. Log value of oil-water (octanol/water) partition coefficient: Uncertain
16. Explosion upper limit (%, V/V): Uncertain
17 . Lower explosion limit (%, V/V): Uncertain
18. Solubility: Insoluble in water, slightly soluble in ethanol, soluble in tetrachloromethane, chloroform, xylene, cyclohexanone and dimethylbenzene Ethyl chloride.
Toxicological data
Acute diurnal LD in rats509000mg/kg, dogs fed industrial products at a dose of 300mg/kg daily did not show symptoms of chronic poisoning for 66 weeks, and the no-effect dose in the 2-year feeding test on rats was 1000mg/kg.
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 66.13
2. Molar volume (cm3/mol): 184.4
3. Isotonic specific volume (90.2K): 517.0
4. Surface tension (3.0 dyne/cm): 61.8
5. Polarizability (0.5 10 -24cm3): 26.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 62.7
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 340
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 2
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
White crystal. Odorless. Solubility at 26°C (g/100ml): chloroform 7.78, tetrachloroethane 8.15, cyclohexanone 4.96, dioxane 4.70, benzene 2.13, toluene 0.69, heptane 0.04, ethanol 0.29, ether 0.25. Almost insoluble in water. Relative density 1.74. Melting point 178℃. The median lethal dose (rat, oral) is 9000mg/kg
It has an irritating effect on the skin and membrane.
Storage method
1. Seal and store.
Synthesis method
1. Preparation of 1,2,3,4-tetrahydrophthalic anhydride. Mix maleic anhydride and benzene and heat to dissolve the maleic anhydride. , when the temperature rises to 70°C, introduce butadiene and react at (75±2)°C until the aeration reaction reaches the end point. Then put the material into the crystallization tank while hot, cool to room temperature, filter, and filter the filter cake at 50 Dry at ~70°C to obtain 1,2,3,4-tetrahydrophthalic anhydride with a content of more than 95%.
2.Preparation of 1,2,3,4-tetrahydrophthalimide Add ammonia water to the reaction bottle , add 1,2,3,4- tetrahydrophthalic anhydride below 50℃, and gradually raise the temperature to 105~110℃ to make the remaining The free ammonia and water evaporate, then gradually heat up to 230~250℃, about 5~5h, release the material, cool to 80℃, dissolve in heated water, cool and crystallize, filter, and dry to obtain1,2,3,4-tetrahydrophthalimide.
3.The synthesis of captan will1,2,3 ,4-Tetrahydrophthalimine is added to 5% to 6% sodium hydroxide solution pre-cooled to 0°C. After it is completely dissolved, add trichlorosulfur chloromethane and stir at 0~ Reaction at 5°C. When the H value is below 8, the reaction is deemed to be completed, filtered, and the filter cake is washed with water until neutral, dried, and Captan is obtained.
4.The reaction between butadiene and maleic anhydride produces 1,2,3,6-tetrahydrobenzene Formic anhydride reacts with ammonia to synthesize 1,2,3,6-tetrahydrophthalimide, and finally reacts with trichlorosulfur chloromethane to obtain captan.
Purpose
1. Used for pesticide analysis standards. Protective fungicide. Antibacterial agents in soap.
2.Trichloromethylthio broad-spectrum protective fungicide, effective on barley, wheat, oats, rice, corn, cotton, and vegetables It has good control effects on many diseases of crops such as , fruit trees, melons, and tobacco. It is safe for crops, non-toxic, and also has the effect of stimulating plant growth. It also has good control effects on rice sheath blight, rice blast, wheat stem rust, tobacco leaf spot disease, cotton seedling disease, apple rot, etc.
3.The maximum allowable content (mass fraction) in cosmetics is 0.5%, mainly used as antibacterial agents in soaps and shampoos , used as fungicides and fungicides in agriculture.
