Hexanoyl chloride
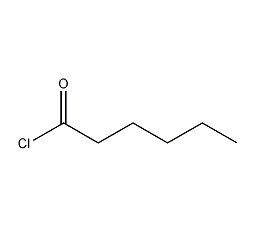

Structural formula
| Business number | 03V5 |
|---|---|
| Molecular formula | C6H11ClO |
| Molecular weight | 134.60 |
| label |
crystalline calcium oxide, Caproyl chloride |
Numbering system
CAS number:142-61-0
MDL number:MFCD00000760
EINECS number:205-549-1
RTECS number:None
BRN number:506332
PubChem number:24849650
Physical property data
1. Physical property data:
1.Character: colorless or light yellow liquid
2.Relative density: 0.9805
3.Refractive index: 1.4286
4.Flash point (℃):50
5.Melting point (℃): -87.3
6.Boiling point (ºC): 151-153
7.Solubility: Soluble in most organic solvents such as ether and chloroform.
Toxicological data
None
Ecological data
3. Ecology Data:
1, other harmful effects: This substance may be harmful to the environment, special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index:34.72
2. Molar volume (cm3/mol): 136.0
3. Isotonic specific volume (90.2K ):314.3
4. Surface tension (dyne/ cm):28.4
5. Polarizability(10-24cm3):13.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 70.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials:Water, alcohols, strong oxidants, strong bases.
Storage method
Store sealed in a cool and dry place.
Synthesis method
Obtained from the reaction of n-ethanol and thionyl chloride. Add thionyl chloride to 50-70℃ mixed with n-hexanoic acid at 70-90℃ reaction 4h. Distilled, collected147-160℃ fraction to obtain hexanoyl chloride.
Purpose
1.This product is used as an intermediate in organic synthesis. Used to prepare the antifungal drug tribromophenyl hexanoate and also used as a liquid crystal intermediate.
2.For organic synthesis.
3.Used as an acylating agent in organic synthesis.
T-SIZE: 9pt; FONT-FAMILY: 宋体; mso-bidi-font-family: Arial; mso-ascii-font-family: Arial; mso-hansi-font-family: Arial”>Polarizability (10-24 cm3):13.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 70.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials:Water, alcohols, strong oxidants, strong bases.
Storage method
Store sealed in a cool and dry place.
Synthesis method
Obtained from the reaction of n-ethanol and thionyl chloride. Add thionyl chloride to 50-70℃ mixed with n-hexanoic acid at 70-90℃ reaction 4h. Distilled, collected147-160℃ fraction to obtain hexanoyl chloride.
Purpose
1.This product is used as an intermediate in organic synthesis. Used to prepare the antifungal drug tribromophenyl hexanoate and also used as a liquid crystal intermediate.
2.For organic synthesis.
3.Used as an acylating agent in organic synthesis.
