Tetrahydropyran
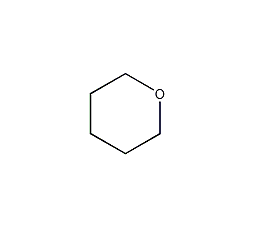

Structural formula
| Business number | 03V9 |
|---|---|
| Molecular formula | C5H10O |
| Molecular weight | 86.13 |
| label |
oxyhexane, Pentamethylene oxide, 1,5-Oxidopentane, Solvent for nitro spray paint, solvent for rubber, Solvent for Grignard reaction |
Numbering system
CAS number:142-68-7
MDL number:MFCD00006585
EINECS number:205-552-8
RTECS number:None
BRN number:None
PubChem number:24857635
Physical property data
1. Properties: colorless, flowing liquid with an ether-like odor.
2. Boiling point (ºC, 101.3kPa): 88
3. Melting point (ºC): -44.2
4. Relative density (g/mL, 15/4ºC): 0.8855
5. Relative density (g/mL, 20/4ºC): 0.8810
6. Relative vapor density (g/mL, air=1): 4.0
7. Refractive index (n20ºC): 1.4200
8. Flash point (ºC): -20
9. Solubility: compatible with water, Miscible with ethanol, ether and general organic solvents. High solubility in resins and rubbers. As a solvent for nitrocellulose, a transparent coating film can be obtained.
10. Relative density (25℃, 4℃): 0.878
11. Critical temperature (ºC): 298.85
12. Critical pressure (MPa) : 4.77
13. Critical density (g·cm-3): 0.328
14. Critical volume (cm3 ·mol-1): 263
15. Critical compression factor: 0.264
16. Eccentricity factor: 0.218
17. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3173.31
18. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -223.38
19. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3138.4
20. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -258.3
Toxicological data
The vapor is narcotic.
Ecological data
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 24.66
2. Molar volume (cm3/mol): 97.4
3. Isotonic specific volume (90.2K ): 224.8
4. Surface tension (dyne/cm): 28.2
5. Polarizability (10-24cm3): 9.77
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 9.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 30.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure. Explosive peroxides can be formed during storage, so reducing agents such as sodium bisulfite and stannous chloride are often added to inhibit the formation of peroxides. Reacts violently with acidic substances. It is unstable when heated and pressurized, so care should be taken when using it.
Chemical properties: Tetrahydropyran is chemically inactive like aliphatic ethers and can generate peroxide under the action of oxygen in the air. Light promotes the formation of peroxide, which reacts with chlorine to obtain various chlorinated tetrahydropyrans. Reacts with acid chloride to form ω-chloropentyl ester. Reacts with ammonia, aliphatic amines, and aromatic amines to obtain piperidine or piperidine substitutes.
Storage method
Store sealed in a cool place.
Synthesis method
None
Purpose
Used as a solvent for nitrocellulose spray paint, rubber, etc. and a solvent for Grignard reaction.
